Abstract
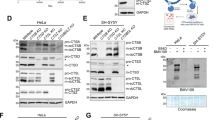
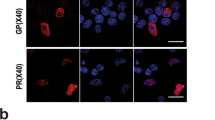
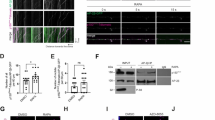
The cell-to-cell transmission of pathological α-synuclein (α-syn) has been proposed to be a critical event in the development of synucleinopathies. Recent studies have begun to reveal the underlying molecular mechanism of α-syn propagation. As one of the central steps, α-syn secretion is reported to be Ca2+-dependent and mediated by unconventional exocytosis. However, the soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) requirement and vesicle identity of α-syn secretion remain elusive. Here we found that α-syn secretion is SNARE-dependent by systematically knocking down Q-SNAREs and R-SNAREs in exocytosis pathways. α-Syn secretion was mainly mediated by syntaxin 4 (STX4) and synaptosomal-associated protein 23 (SNAP23), but did not require STX1 and SNAP25, in differentiated SH-SY5Y cells. On the other hand, vesicle-associated membrane protein 3 (VAMP3), VAMP7, and VAMP8 were all involved in α-syn secretion, most likely in overlapping pathways. Application of super-resolution microscopy revealed localization of both endogenous and overexpressed α-syn in endosomes, lysosomes, and autophagosomes in rat primary cortical neurons. α-Syn co-localized with microtubule-associated protein 1 light chain 3 (LC3) most extensively, suggesting its tight association with the autophagy pathway. Consistently, α-syn secretion was regulated by the autophagy-lysosome pathway. Collectively, our data suggest that α-syn secretion is SNARE-dependent and is mediated by multiple vesicular pathways including exocytosis of recycling endosomes, multivesicular bodies, autophagosomes, and lysosomes.






Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this manuscript.
Code Availability
Not applicable.
Abbreviations
- α-syn:
-
Alpha-synuclein
- BafA1:
-
Bafilomycin A1
- BAPTA-AM:
-
1,2-Bis(o-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid acetoxymethyl ester
- BFA:
-
Brefeldin A
- CQ:
-
Chloroquine
- DMSO:
-
Dimethyl sulfoxide
- EDTA:
-
Ethylene diamine tetraacetic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- Hrs:
-
Hepatocyte growth factor-regulated tyrosine kinase substrate
- KD:
-
Knockdown
- LC3:
-
Microtubule-associated protein 1 light chain 3
- LDH:
-
Lactate dehydrogenase
- 3-MA:
-
3-Methyladenine
- MVBs:
-
Multivesicular bodies
- PD:
-
Parkinson’s disease
- PM:
-
Plasma membrane
- Rab:
-
Member RAS oncogene family
- SNARE:
-
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- SNAP:
-
Synaptosomal-associated protein
- STX:
-
Syntaxin
- TG:
-
Thapsigargin
- VAMP:
-
Vesicle-associated membrane protein
References
Maroteaux L, Campanelli JT, Scheller RH (1988) Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci 8(8):2804–2815
Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M (1998) Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett 251(3):205–208. https://doi.org/10.1016/s0304-3940(98)00504-7
Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M (1998) alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci U S A 95(11):6469–6473. https://doi.org/10.1073/pnas.95.11.6469
Tateno F, Sakakibara R, Kawai T, Kishi M, Murano T (2012) Alpha-synuclein in the cerebrospinal fluid differentiates synucleinopathies (Parkinson disease, dementia with Lewy bodies, multiple system atrophy) from Alzheimer disease. Alzheimer Dis Assoc Disord 26(3):213–216. https://doi.org/10.1097/WAD.0b013e31823899cc
El-Agnaf OM, Salem SA, Paleologou KE, Cooper LJ, Fullwood NJ, Gibson MJ, Curran MD, Court JA et al (2003) Alpha-synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. FASEB J 17(13):1945–1947. https://doi.org/10.1096/fj.03-0098fje
Mollenhauer B, Trautmann E, Taylor P, Manninger P, Sixel-Doring F, Ebentheuer J, Trenkwalder C, Schlossmacher MG (2013) Total CSF alpha-synuclein is lower in de novo Parkinson patients than in healthy subjects. Neurosci Lett 532:44–48. https://doi.org/10.1016/j.neulet.2012.11.004
Kang UJ, Boehme AK, Fairfoul G, Shahnawaz M, Ma TC, Hutten SJ, Green A, Soto C (2019) Comparative study of cerebrospinal fluid alpha-synuclein seeding aggregation assays for diagnosis of Parkinson’s disease. Mov Disord 34(4):536–544. https://doi.org/10.1002/mds.27646
Christensen DP, Ejlerskov P, Rasmussen I, Vilhardt F (2016) Reciprocal signals between microglia and neurons regulate alpha-synuclein secretion by exophagy through a neuronal cJUN-N-terminal kinase-signaling axis. J Neuroinflammation 13(1):59. https://doi.org/10.1186/s12974-016-0519-5
Jang A, Lee HJ, Suk JE, Jung JW, Kim KP, Lee SJ (2010) Non-classical exocytosis of alpha-synuclein is sensitive to folding states and promoted under stress conditions. J Neurochem 113(5):1263–1274. https://doi.org/10.1111/j.1471-4159.2010.06695.x
Lee HJ, Patel S, Lee SJ (2005) Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci 25(25):6016–6024. https://doi.org/10.1523/JNEUROSCI.0692-05.2005
Mahul-Mellier AL, Vercruysse F, Maco B, Ait-Bouziad N, De Roo M, Muller D, Lashuel HA (2015) Fibril growth and seeding capacity play key roles in alpha-synuclein-mediated apoptotic cell death. Cell Death Differ 22(12):2107–2122. https://doi.org/10.1038/cdd.2015.79
Lee HJ, Bae EJ, Lee SJ (2014) Extracellular alpha-synuclein-a novel and crucial factor in Lewy body diseases. Nat Rev Neurol 10(2):92–98. https://doi.org/10.1038/nrneurol.2013.275
Hansen C, Li JY (2012) Beyond alpha-synuclein transfer: pathology propagation in Parkinson’s disease. Trends Mol Med 18(5):248–255. https://doi.org/10.1016/j.molmed.2012.03.002
Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB (2005) Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflammation 2:14. https://doi.org/10.1186/1742-2094-2-14
Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W et al (2005) Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J 19(6):533–542. https://doi.org/10.1096/fj.04-2751com
Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ (2008) Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol Aging 29(11):1690–1701. https://doi.org/10.1016/j.neurobiolaging.2007.04.006
Klegeris A, Giasson BI, Zhang H, Maguire J, Pelech S, McGeer PL (2006) Alpha-synuclein and its disease-causing mutants induce ICAM-1 and IL-6 in human astrocytes and astrocytoma cells. FASEB J 20(12):2000–2008. https://doi.org/10.1096/fj.06-6183com
Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K (2010) Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci 30(20):6838–6851. https://doi.org/10.1523/JNEUROSCI.5699-09.2010
Ariesandi W, Chang CF, Chen TE, Chen YR (2013) Temperature-dependent structural changes of Parkinson’s alpha-synuclein reveal the role of pre-existing oligomers in alpha-synuclein fibrillization. PLoS ONE 8(1):e53487. https://doi.org/10.1371/journal.pone.0053487
Lee HJ, Suk JE, Bae EJ, Lee JH, Paik SR, Lee SJ (2008) Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol 40(9):1835–1849. https://doi.org/10.1016/j.biocel.2008.01.017
Liu J, Zhang JP, Shi M, Quinn T, Bradner J, Beyer R, Chen S, Zhang J (2009) Rab11a and HSP90 regulate recycling of extracellular alpha-synuclein. J Neurosci 29(5):1480–1485. https://doi.org/10.1523/JNEUROSCI.6202-08.2009
Verweij FJ, Middeldorp JM, Pegtel DM (2012) Intracellular signaling controlled by the endosomal-exosomal pathway. Commun Integr Biol 5(1):88–93
Lo Cicero A, Stahl PD, Raposo G (2015) Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr Opin Cell Biol 35:69–77. https://doi.org/10.1016/j.ceb.2015.04.013
Kong SM, Chan BK, Park JS, Hill KJ, Aitken JB, Cottle L, Farghaian H, Cole AR et al (2014) Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes alpha-synuclein externalization via exosomes. Hum Mol Genet 23(11):2816–2833. https://doi.org/10.1093/hmg/ddu099
Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ, Cooper JM (2011) Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis 42(3):360–367. https://doi.org/10.1016/j.nbd.2011.01.029
Hasegawa T, Konno M, Baba T, Sugeno N, Kikuchi A, Kobayashi M, Miura E, Tanaka N et al (2011) The AAA-ATPase VPS4 regulates extracellular secretion and lysosomal targeting of alpha-synuclein. PLoS ONE 6(12):e29460. https://doi.org/10.1371/journal.pone.0029460
Poehler AM, Xiang W, Spitzer P, May VE, Meixner H, Rockenstein E, Chutna O, Outeiro TF et al (2014) Autophagy modulates SNCA/alpha-synuclein release, thereby generating a hostile microenvironment. Autophagy 10(12):2171–2192. https://doi.org/10.4161/auto.36436
Minakaki G, Menges S, Kittel A, Emmanouilidou E, Schaeffner I, Barkovits K, Bergmann A, Rockenstein E et al (2018) Autophagy inhibition promotes SNCA/alpha-synuclein release and transfer via extracellular vesicles with a hybrid autophagosome-exosome-like phenotype. Autophagy 14(1):98–119. https://doi.org/10.1080/15548627.2017.1395992
Vogiatzi T, Xilouri M, Vekrellis K, Stefanis L (2008) Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J Biol Chem 283(35):23542–23556. https://doi.org/10.1074/jbc.M801992200
Lee HJ, Cho ED, Lee KW, Kim JH, Cho SG, Lee SJ (2013) Autophagic failure promotes the exocytosis and intercellular transfer of alpha-synuclein. Exp Mol Med 45:e22. https://doi.org/10.1038/emm.2013.45
Fussi N, Hollerhage M, Chakroun T, Nykanen NP, Rosler TW, Koeglsperger T, Wurst W, Behrends C et al (2018) Exosomal secretion of alpha-synuclein as protective mechanism after upstream blockage of macroautophagy. Cell Death Dis 9(7):757. https://doi.org/10.1038/s41419-018-0816-2
Ejlerskov P, Rasmussen I, Nielsen TT, Bergstrom AL, Tohyama Y, Jensen PH, Vilhardt F (2013) Tubulin polymerization-promoting protein (TPPP/p25alpha) promotes unconventional secretion of alpha-synuclein through exophagy by impairing autophagosome-lysosome fusion. J Biol Chem 288(24):17313–17335. https://doi.org/10.1074/jbc.M112.401174
Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE (1998) SNAREpins: minimal machinery for membrane fusion. Cell 92(6):759–772
Jahn R, Scheller RH (2006) SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol 7(9):631–643. https://doi.org/10.1038/nrm2002
Stow JL, Manderson AP, Murray RZ (2006) SNAREing immunity: the role of SNAREs in the immune system. Nat Rev Immunol 6(12):919–929. https://doi.org/10.1038/nri1980
Hong W (2005) SNAREs and traffic. Biochim Biophys Acta 1744(3):493–517
Dingjan I, Linders PTA, Verboogen DRJ, Revelo NH, Ter Beest M, van den Bogaart G (2018) Endosomal and phagosomal SNAREs. Physiol Rev 98(3):1465–1492. https://doi.org/10.1152/physrev.00037.2017
Guan Y, Zhao X, Liu F, Yan S, Wang Y, Du C, Cui X, Li R et al (2020) Pathogenic mutations differentially regulate cell-to-cell transmission of alpha-synuclein. Front Cell Neurosci 14:159. https://doi.org/10.3389/fncel.2020.00159
Du C, Wang Y, Zhang F, Yan S, Guan Y, Gong X, Zhang T, Cui X et al (2017) Synaptotagmin-11 inhibits cytokine secretion and phagocytosis in microglia. Glia 65(10):1656–1667. https://doi.org/10.1002/glia.23186
Yan S, Wang Y, Zhang Y, Wang L, Zhao X, Du C, Gao P, Yan F et al (2020) Synaptotagmin-11 regulates the functions of caveolae and responds to mechanical stimuli in astrocytes. FASEB J 34(2):2609–2624. https://doi.org/10.1096/fj.201901715R
Oh SH, Kim HN, Park HJ, Shin JY, Bae EJ, Sunwoo MK, Lee SJ, Lee PH (2016) Mesenchymal stem cells inhibit transmission of alpha-synuclein by modulating clathrin-mediated endocytosis in a parkinsonian model. Cell Rep 14(4):835–849. https://doi.org/10.1016/j.celrep.2015.12.075
Shin OH (2014) Exocytosis and synaptic vesicle function. Compr Physiol 4(1):149–175. https://doi.org/10.1002/cphy.c130021
Park JJ, Gondre-Lewis MC, Eiden LE, Loh YP (2011) A distinct trans-Golgi network subcompartment for sorting of synaptic and granule proteins in neurons and neuroendocrine cells. J Cell Sci 124(Pt 5):735–744. https://doi.org/10.1242/jcs.076372
Jahn R, Fasshauer D (2012) Molecular machines governing exocytosis of synaptic vesicles. Nature 490(7419):201–207. https://doi.org/10.1038/nature11320
Veale KJ, Offenhauser C, Lei N, Stanley AC, Stow JL, Murray RZ (2011) VAMP3 regulates podosome organisation in macrophages and together with Stx4/SNAP23 mediates adhesion, cell spreading and persistent migration. Exp Cell Res 317(13):1817–1829. https://doi.org/10.1016/j.yexcr.2011.04.016
Chiaruttini G, Piperno GM, Jouve M, De Nardi F, Larghi P, Peden AA, Baj G, Muller S et al (2016) The SNARE VAMP7 regulates exocytic trafficking of interleukin-12 in dendritic cells. Cell Rep 14(11):2624–2636. https://doi.org/10.1016/j.celrep.2016.02.055
Sander LE, Frank SP, Bolat S, Blank U, Galli T, Bigalke H, Bischoff SC, Lorentz A (2008) Vesicle associated membrane protein (VAMP)-7 and VAMP-8, but not VAMP-2 or VAMP-3, are required for activation-induced degranulation of mature human mast cells. Eur J Immunol 38(3):855–863. https://doi.org/10.1002/eji.200737634
Nishioka K, Hayashi S, Farrer MJ, Singleton AB, Yoshino H, Imai H, Kitami T, Sato K et al (2006) Clinical heterogeneity of alpha-synuclein gene duplication in Parkinson’s disease. Ann Neurol 59(2):298–309. https://doi.org/10.1002/ana.20753
Xu H, Arnold MG, Kumar SV (2015) Differential effects of Munc18s on multiple degranulation-relevant trans-SNARE complexes. PLoS ONE 10(9):e0138683. https://doi.org/10.1371/journal.pone.0138683
Proux-Gillardeaux V, Galli T, Callebaut I, Mikhailik A, Calothy G, Marx M (2003) D53 is a novel endosomal SNARE-binding protein that enhances interaction of syntaxin 1 with the synaptobrevin 2 complex in vitro. Biochem J 370(Pt 1):213–221. https://doi.org/10.1042/BJ20021309
Veale KJ, Offenhauser C, Murray RZ (2011) The role of the recycling endosome in regulating lamellipodia formation and macrophage migration. Commun Integr Biol 4(1):44–47. https://doi.org/10.4161/cib.4.1.13569
Wei Y, Wang D, Jin F, Bian Z, Li L, Liang H, Li M, Shi L et al (2017) Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23. Nat Commun 8:14041. https://doi.org/10.1038/ncomms14041
Williams KC, McNeilly RE, Coppolino MG (2014) SNAP23, Syntaxin4, and vesicle-associated membrane protein 7 (VAMP7) mediate trafficking of membrane type 1-matrix metalloproteinase (MT1-MMP) during invadopodium formation and tumor cell invasion. Mol Biol Cell 25(13):2061–2070. https://doi.org/10.1091/mbc.E13-10-0582
Dolai S, Liang T, Lam PPL, Fernandez NA, Chidambaram S, Gaisano HY (2012) Effects of ethanol metabolites on exocytosis of pancreatic acinar cells in rats. Gastroenterology 143(3):832-843 e837. https://doi.org/10.1053/j.gastro.2012.06.011
Zhou H, Wei Z, Wang S, Yao D, Zhang R, Ma C (2019) Structural and functional analysis of the CAPS SNARE-binding domain required for SNARE complex formation and exocytosis. Cell Rep 26(12):3347-3359 e3346. https://doi.org/10.1016/j.celrep.2019.02.064
Nakata Y, Yasuda T, Fukaya M, Yamamori S, Itakura M, Nihira T, Hayakawa H, Kawanami A et al (2012) Accumulation of alpha-synuclein triggered by presynaptic dysfunction. J Neurosci 32(48):17186–17196. https://doi.org/10.1523/JNEUROSCI.2220-12.2012
Murray RZ, Kay JG, Sangermani DG, Stow JL (2005) A role for the phagosome in cytokine secretion. Science 310(5753):1492–1495. https://doi.org/10.1126/science.1120225
Puri C, Renna M, Bento CF, Moreau K, Rubinsztein DC (2014) ATG16L1 meets ATG9 in recycling endosomes: additional roles for the plasma membrane and endocytosis in autophagosome biogenesis. Autophagy 10(1):182–184. https://doi.org/10.4161/auto.27174
Kehl A, Goser V, Reuter T, Liss V, Franke M, John C, Richter CP, Deiwick J et al (2020) A trafficome-wide RNAi screen reveals deployment of early and late secretory host proteins and the entire late endo-/lysosomal vesicle fusion machinery by intracellular Salmonella. PLoS Pathog 16(7):e1008220. https://doi.org/10.1371/journal.ppat.1008220
Fader CM, Sanchez DG, Mestre MB, Colombo MI (2009) TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta 1793(12):1901–1916. https://doi.org/10.1016/j.bbamcr.2009.09.011
Galli T, Zahraoui A, Vaidyanathan VV, Raposo G, Tian JM, Karin M, Niemann H, Louvard D (1998) A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol Biol Cell 9(6):1437–1448
Jani RA, Purushothaman LK, Rani S, Bergam P, Setty SR (2015) STX13 regulates cargo delivery from recycling endosomes during melanosome biogenesis. J Cell Sci 128(17):3263–3276. https://doi.org/10.1242/jcs.171165
Fader CM, Aguilera MO, Colombo MI (2012) ATP is released from autophagic vesicles to the extracellular space in a VAMP7-dependent manner. Autophagy 8(12):1741–1756. https://doi.org/10.4161/auto.21858
Moreau K, Ravikumar B, Renna M, Puri C, Rubinsztein DC (2011) Autophagosome precursor maturation requires homotypic fusion. Cell 146(2):303–317. https://doi.org/10.1016/j.cell.2011.06.023
Pols MS, van Meel E, Oorschot V, ten Brink C, Fukuda M, Swetha MG, Mayor S, Klumperman J (2013) hVps41 and VAMP7 function in direct TGN to late endosome transport of lysosomal membrane proteins. Nat Commun 4:1361. https://doi.org/10.1038/ncomms2360
Advani RJ, Yang B, Prekeris R, Lee KC, Klumperman J, Scheller RH (1999) VAMP-7 mediates vesicular transport from endosomes to lysosomes. J Cell Biol 146(4):765–776
Rao SK, Huynh C, Proux-Gillardeaux V, Galli T, Andrews NW (2004) Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J Biol Chem 279(19):20471–20479. https://doi.org/10.1074/jbc.M400798200
Marshall MR, Pattu V, Halimani M, Maier-Peuschel M, Muller ML, Becherer U, Hong W, Hoth M et al (2015) VAMP8-dependent fusion of recycling endosomes with the plasma membrane facilitates T lymphocyte cytotoxicity. J Cell Biol 210(1):135–151. https://doi.org/10.1083/jcb.201411093
Nair-Gupta P, Baccarini A, Tung N, Seyffer F, Florey O, Huang Y, Banerjee M, Overholtzer M et al (2014) TLR signals induce phagosomal MHC-I delivery from the endosomal recycling compartment to allow cross-presentation. Cell 158(3):506–521. https://doi.org/10.1016/j.cell.2014.04.054
Becken U, Jeschke A, Veltman K, Haas A (2010) Cell-free fusion of bacteria-containing phagosomes with endocytic compartments. Proc Natl Acad Sci U S A 107(48):20726–20731. https://doi.org/10.1073/pnas.1007295107
Itakura E, Kishi-Itakura C, Mizushima N (2012) The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 151(6):1256–1269. https://doi.org/10.1016/j.cell.2012.11.001
Shui W, Sheu L, Liu J, Smart B, Petzold CJ, Hsieh TY, Pitcher A, Keasling JD et al (2008) Membrane proteomics of phagosomes suggests a connection to autophagy. Proc Natl Acad Sci U S A 105(44):16952–16957. https://doi.org/10.1073/pnas.0809218105
Antonin W, Holroyd C, Fasshauer D, Pabst S, Von Mollard GF, Jahn R (2000) A SNARE complex mediating fusion of late endosomes defines conserved properties of SNARE structure and function. EMBO J 19(23):6453–6464. https://doi.org/10.1093/emboj/19.23.6453
Antonin W, Holroyd C, Tikkanen R, Honing S, Jahn R (2000) The R-SNARE endobrevin/VAMP-8 mediates homotypic fusion of early endosomes and late endosomes. Mol Biol Cell 11(10):3289–3298. https://doi.org/10.1091/mbc.11.10.3289
Pryor PR, Mullock BM, Bright NA, Lindsay MR, Gray SR, Richardson SC, Stewart A, James DE et al (2004) Combinatorial SNARE complexes with VAMP7 or VAMP8 define different late endocytic fusion events. EMBO Rep 5(6):590–595. https://doi.org/10.1038/sj.embor.7400150
Polgar J, Chung SH, Reed GL (2002) Vesicle-associated membrane protein 3 (VAMP-3) and VAMP-8 are present in human platelets and are required for granule secretion. Blood 100(3):1081–1083
Schwarz L, Goldbaum O, Bergmann M, Probst-Cousin S, Richter-Landsberg C (2012) Involvement of macroautophagy in multiple system atrophy and protein aggregate formation in oligodendrocytes. J Mol Neurosci 47(2):256–266. https://doi.org/10.1007/s12031-012-9733-5
Kumar ST, Donzelli S, Chiki A, Syed MMK, Lashuel HA (2020) A simple, versatile and robust centrifugation-based filtration protocol for the isolation and quantification of alpha-synuclein monomers, oligomers and fibrils: towards improving experimental reproducibility in alpha-synuclein research. J Neurochem 153(1):103–119. https://doi.org/10.1111/jnc.14955
Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG et al (2002) Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 –> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A 99(13):8968–8973. https://doi.org/10.1073/pnas.132197599
Lv Z, Krasnoslobodtsev AV, Zhang Y, Ysselstein D, Rochet JC, Blanchard SC, Lyubchenko YL (2016) Effect of acidic pH on the stability of alpha-synuclein dimers. Biopolymers 105(10):715–724. https://doi.org/10.1002/bip.22874
Buell AK, Galvagnion C, Gaspar R, Sparr E, Vendruscolo M, Knowles TP, Linse S, Dobson CM (2014) Solution conditions determine the relative importance of nucleation and growth processes in alpha-synuclein aggregation. Proc Natl Acad Sci U S A 111(21):7671–7676. https://doi.org/10.1073/pnas.1315346111
Acknowledgements
We thank Profs. Xiaomin Wang and Zhi-Qing David Xu for their valuable comments on this project and Pei Gao for drafting the schematic diagram.
Funding
This work was supported by Beijing Natural Science Foundation Program and Scientific Research Key Program of Beijing Municipal Commission of Education (KZ201510025023); the National Natural Science Foundation of China (31471085, 91849103, 81671248); and Chaoyang District Science and Technology Plan Project (CYSF-1933).
Author information
Authors and Affiliations
Contributions
C. X. Zhang and R. Li designed research; X. Zhao, Y. Guan, F. Liu, S. Yan, Y. Wang, M. Hu, and Y. Li performed experiments; X. Zhao and Y. Guan analyzed data; and C. X. Zhang, X. Zhao, and R. Li wrote the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaofang Zhao, Yuan Guan, and Fengwei Liu contributed equally to the work.
Rights and permissions
About this article
Cite this article
Zhao, X., Guan, Y., Liu, F. et al. SNARE Proteins Mediate α-Synuclein Secretion via Multiple Vesicular Pathways. Mol Neurobiol 59, 405–419 (2022). https://doi.org/10.1007/s12035-021-02599-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02599-0