Abstract
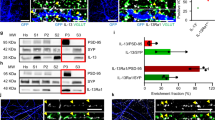
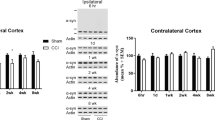
Traumatic brain injury (TBI) can produce lasting cognitive, emotional, and somatic difficulties that can impact quality of life for patients living with an injury. Impaired hippocampal function and synaptic alterations have been implicated in contributing to cognitive difficulties in experimental TBI models. In the synapse, neuronal communication is facilitated by the regulated release of neurotransmitters from docking presynaptic vesicles. The synaptic vesicle glycoprotein 2 (SV2) isoforms SV2A and SV2B play central roles in the maintenance of the readily releasable pool of vesicles and the coupling of calcium to the N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex responsible for vesicle docking. Recently, we reported the findings of TBI-induced reductions in presynaptic vesicle density and SNARE complex formation; however, the effect of TBI on SV2 is unknown. To investigate this, rats were subjected to controlled cortical impact (CCI) or sham control surgery. Abundance of SV2A and SV2B were assessed at 1, 3, 7, and 14 days post-injury by immunoblot. SV2A and SV2B were reduced in the cortex at several time points and in the hippocampus at every time point assessed. Immunohistochemical staining and quantitative intensity measurements completed at 14 days post-injury revealed reduced SV2A immunoreactivity in all hippocampal subregions and reduced SV2B immunoreactivity in the molecular layer after CCI. Reductions in SV2A abundance and immunoreactivity occurred concomitantly with motor dysfunction and spatial learning and memory impairments in the 2 weeks post-injury. These findings provide novel evidence for the effect of TBI on SV2 with implications for impaired neurotransmission neurobehavioral dysfunction after TBI.








Similar content being viewed by others
Data Availability
All data generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Pierce JE, Smith DH, Trojanowski JQ, McIntosh TK (1998) Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience 87(2):359–369
Lundin A, de Boussard C, Edman G, Borg J (2006) Symptoms and disability until 3 months after mild TBI. Brain Inj 20(8):799–806. https://doi.org/10.1080/02699050600744327
Truelle JL, Koskinen S, Hawthorne G, Sarajuuri J, Formisano R, Von Wild K, Neugebauer E, Wilson L et al (2010) Quality of life after traumatic brain injury: the clinical use of the QOLIBRI, a novel disease-specific instrument. Brain Inj 24(11):1272–1291. https://doi.org/10.3109/02699052.2010.506865
Levin HS (1998) Cognitive function outcomes after traumatic brain injury. Curr Opin Neurol 11(6):643–646
Wright MJ, Monti MM, Lutkenhoff ES, Hardy DJ, Litvin PY, Kelly DF, Guskiewicz K, Cantu RC et al (2020) Memory in repeat sports-related concussive injury and single-impact traumatic brain injury. Brain Inj 34(12):1666–1673. https://doi.org/10.1080/02699052.2020.1825806
Agtarap SD, Campbell-Sills L, Jain S, Sun X, Dikmen S, Levin H, McCrea M, Mukherjee P et al (2020) Satisfaction with life following mild traumatic brain injury: a TRACK-TBI Study. J Neurotrauma. https://doi.org/10.1089/neu.2020.7055
Folweiler KA, Samuel S, Metheny HE, Cohen AS (2018) Diminished dentate gyrus filtering of cortical input leads to enhanced area Ca3 excitability after mild traumatic brain injury. J Neurotrauma 35(11):1304–1317. https://doi.org/10.1089/neu.2017.5350
Folweiler KA, Xiong G, Best KM, Metheny HE, Nah G, Cohen AS (2020) Traumatic brain injury diminishes feedforward activation of parvalbumin-expressing interneurons in the dentate gyrus. eNeuro 7(6). https://doi.org/10.1523/ENEURO.0195-19.2020
Norris CM, Scheff SW (2009) Recovery of afferent function and synaptic strength in hippocampal CA1 following traumatic brain injury. J Neurotrauma 26(12):2269–2278. https://doi.org/10.1089/neu.2009.1029
Hunt RF, Scheff SW, Smith BN (2009) Posttraumatic epilepsy after controlled cortical impact injury in mice. Exp Neurol 215(2):243–252. https://doi.org/10.1016/j.expneurol.2008.10.005
Hunt RF, Scheff SW, Smith BN (2011) Synaptic reorganization of inhibitory hilar interneuron circuitry after traumatic brain injury in mice. J Neurosci 31(18):6880–6890. https://doi.org/10.1523/JNEUROSCI.0032-11.2011
Reeves TM, Lyeth BG, Phillips LL, Hamm RJ, Povlishock JT (1997) The effects of traumatic brain injury on inhibition in the hippocampus and dentate gyrus. Brain Res 757(1):119–132
Wolf JA, Johnson BN, Johnson VE, Putt ME, Browne KD, Mietus CJ, Brown DP, Wofford KL et al (2017) Concussion induces hippocampal circuitry disruption in swine. J Neurotrauma 34(14):2303–2314. https://doi.org/10.1089/neu.2016.4848
Beitchman JA, Griffiths DR, Hur Y, Ogle SB, Bromberg CE, Morrison HW, Lifshitz J, Adelson PD et al (2019) Experimental traumatic brain injury induces chronic glutamatergic dysfunction in amygdala circuitry known to regulate anxiety-like behavior. Front Neurosci 13:1434. https://doi.org/10.3389/fnins.2019.01434
Wagner AK, Drewencki LL, Chen X, Santos FR, Khan AS, Harun R, Torres GE, Michael AC, Dixon CE (2009) Chronic methylphenidate treatment enhances striatal dopamine neurotransmission after experimental traumatic brain injury. J Neurochem 108(4):986–997. https://doi.org/10.1111/j.1471-4159.2008.05840.x
Hunt RF, Scheff SW, Smith BN (2010) Regionally localized recurrent excitation in the dentate gyrus of a cortical contusion model of posttraumatic epilepsy. J Neurophysiol 103(3):1490–1500. https://doi.org/10.1152/jn.00957.2009
Bonislawski DP, Schwarzbach EP, Cohen AS (2007) Brain injury impairs dentate gyrus inhibitory efficacy. Neurobiol Dis 25(1):163–169. https://doi.org/10.1016/j.nbd.2006.09.002
Carlson SW, Dixon CE (2018) Lithium improves dopamine neurotransmission and increases dopaminergic protein abundance in the striatum after traumatic brain injury. J Neurotrauma. https://doi.org/10.1089/neu.2017.5509
Carlson SW, Henchir J, Dixon CE (2017) Lateral fluid percussion injury impairs hippocampal synaptic soluble N-ethylmaleimide sensitive factor attachment protein receptor complex formation. Front Neurol 8(532). https://doi.org/10.3389/fneur.2017.00532
Carlson SW, Yan H, Ma M, Li Y, Henchir J, Dixon CE (2016) Traumatic brain injury impairs soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex formation and alters synaptic vesicle distribution in the hippocampus. J Neurotrauma 33(1):113–121. https://doi.org/10.1089/neu.2014.3839
Bajjalieh SM, Frantz GD, Weimann JM, McConnell SK, Scheller RH (1994) Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neurosci 14(9):5223–5235
Chang WP, Sudhof TC (2009) SV2 renders primed synaptic vesicles competent for Ca2+ -induced exocytosis. J Neurosci 29(4):883–897. https://doi.org/10.1523/JNEUROSCI.4521-08.2009
Wan QF, Zhou ZY, Thakur P, Vila A, Sherry DM, Janz R, Heidelberger R (2010) SV2 acts via presynaptic calcium to regulate neurotransmitter release. Neuron 66(6):884–895. https://doi.org/10.1016/j.neuron.2010.05.010
Custer KL, Austin NS, Sullivan JM, Bajjalieh SM (2006) Synaptic vesicle protein 2 enhances release probability at quiescent synapses. J Neurosci 26(4):1303–1313. https://doi.org/10.1523/JNEUROSCI.2699-05.2006
Xu T, Bajjalieh SM (2001) SV2 modulates the size of the readily releasable pool of secretory vesicles. Nat Cell Biol 3(8):691–698. https://doi.org/10.1038/35087000
Bajjalieh SM, Peterson K, Shinghal R, Scheller RH (1992) SV2, a brain synaptic vesicle protein homologous to bacterial transporters. Science 257(5074):1271–1273. https://doi.org/10.1126/science.1519064
Janz R, Goda Y, Geppert M, Missler M, Sudhof TC (1999) SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron 24(4):1003–1016
Feany MB, Lee S, Edwards RH, Buckley KM (1992) The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell 70(5):861–867. https://doi.org/10.1016/0092-8674(92)90319-8
Crevecoeur J, Foerch P, Doupagne M, Thielen C, Vandenplas C, Moonen G, Deprez M, Rogister B (2013) Expression of SV2 isoforms during rodent brain development. BMC Neurosci 14:87. https://doi.org/10.1186/1471-2202-14-87
Bartholome O, Van den Ackerveken P, Sanchez Gil J, de la Brassinne BO, Leprince P, Franzen R, Rogister B (2017) Puzzling out synaptic vesicle 2 family members functions. Front Mol Neurosci 10:148. https://doi.org/10.3389/fnmol.2017.00148
Nowack A, Yao J, Custer KL, Bajjalieh SM (2010) SV2 regulates neurotransmitter release via multiple mechanisms. Am J Physiol Cell Physiol 299(5):C960-967. https://doi.org/10.1152/ajpcell.00259.2010
Carlson SW, Yan H, Dixon CE (2017) Lithium increases hippocampal SNARE protein abundance after traumatic brain injury. Exp Neurol 289:55–63. https://doi.org/10.1016/j.expneurol.2016.12.006
Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL (1991) A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods 39(3):253–262
Paxinos G, Watson C (2009) The rat brain in stereotaxic coordinates. Compact Sixth Edition. Elsevier Inc., London
Dixon CE, Bao J, Long DA, Hayes RL (1996) Reduced evoked release of acetylcholine in the rodent hippocampus following traumatic brain injury. Pharmacol Biochem Behav 53(3):679–686
Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang HZ, Peterson MR, Scheller RH, Chavkin C et al (1999) Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A). Proc Natl Acad Sci USA 96(26):15268–15273
Anderson GD, Peterson TC, Farin FM, Bammler TK, Beyer RP, Kantor ED, Hoane MR (2013) The effect of nicotinamide on gene expression in a traumatic brain injury model. Front Neurosci 7:21. https://doi.org/10.3389/fnins.2013.00021
Kochanek PM, Dixon CE, Shellington DK, Shin SS, Bayir H, Jackson EK, Kagan VE, Yan HQ et al (2013) Screening of biochemical and molecular mechanisms of secondary injury and repair in the brain after experimental blast-induced traumatic brain injury in rats. J Neurotrauma 30(11):920–937. https://doi.org/10.1089/neu.2013.2862
Boone DR, Weisz HA, Willey HE, Torres KEO, Falduto MT, Sinha M, Spratt H, Bolding IJ et al (2019) Traumatic brain injury induces long-lasting changes in immune and regenerative signaling. PLoS ONE 14(4):e0214741. https://doi.org/10.1371/journal.pone.0214741
Butler CR, Boychuk JA, Smith BN (2017) Brain injury-induced synaptic reorganization in hilar inhibitory neurons is differentially suppressed by rapamycin. eNeuro 4(5). https://doi.org/10.1523/ENEURO.0134-17.2017
Kochanek PM, Vagni VA, Janesko KL, Washington CB, Crumrine PK, Garman RH, Jenkins LW, Clark RS et al (2006) Adenosine A1 receptor knockout mice develop lethal status epilepticus after experimental traumatic brain injury. J Cereb Blood Flow Metab 26(4):565–575. https://doi.org/10.1038/sj.jcbfm.9600218
Semple BD, O’Brien TJ, Gimlin K, Wright DK, Kim SE, Casillas-Espinosa PM, Webster KM, Petrou S et al (2017) Interleukin-1 receptor in seizure susceptibility after traumatic injury to the pediatric brain. J Neurosci 37(33):7864–7877. https://doi.org/10.1523/JNEUROSCI.0982-17.2017
Konan LM, Song H, Pentecost G, Fogwe D, Ndam T, Cui J, Johnson CE, Grant D et al (2019) Multi-focal neuronal ultrastructural abnormalities and synaptic alterations in mice after low-intensity blast exposure. J Neurotrauma 36(13):2117–2128. https://doi.org/10.1089/neu.2018.6260
Gronborg M, Pavlos NJ, Brunk I, Chua JJ, Munster-Wandowski A, Riedel D, Ahnert-Hilger G, Urlaub H et al (2010) Quantitative comparison of glutamatergic and GABAergic synaptic vesicles unveils selectivity for few proteins including MAL2, a novel synaptic vesicle protein. J Neurosci 30(1):2–12. https://doi.org/10.1523/JNEUROSCI.4074-09.2010
Frankowski JC, Kim YJ, Hunt RF (2019) Selective vulnerability of hippocampal interneurons to graded traumatic brain injury. Neurobiol Dis 129:208–216. https://doi.org/10.1016/j.nbd.2018.07.022
Sheehan P, Zhu M, Beskow A, Vollmer C, Waites CL (2016) Activity-dependent degradation of synaptic vesicle proteins requires Rab35 and the ESCRT pathway. J Neurosci 36(33):8668–8686. https://doi.org/10.1523/JNEUROSCI.0725-16.2016
Sheehan P, Waites CL (2019) Coordination of synaptic vesicle trafficking and turnover by the Rab35 signaling network. Small GTPases 10(1):54–63. https://doi.org/10.1080/21541248.2016.1270392
Sharma M, Burre J, Sudhof TC (2012) Proteasome inhibition alleviates SNARE-dependent neurodegeneration. Sci Transl Med 4(147):147ra113. https://doi.org/10.1126/scitranslmed.3004028
Sharma M, Burre J, Sudhot TC (2011) CSPalpha promotes SNARE-complex assembly by chaperoning SNAP-25 during synaptic activity. Nat Cell Biol 13(1):30–39. https://doi.org/10.1038/ncb2131
Liu M, Akle V, Zheng W, Tortella FC, Hayes RL, Wang KK (2006) Comparing calpain- and caspase-3-mediated degradation patterns in traumatic brain injury by differential proteome analysis. Biochem J 394(3):715–725. https://doi.org/10.1042/BJ20050905
Carlson SW, Yan HQ, Li Y, Henchir J, Ma X, Young MS, Ikonomovic MD, Dixon CE (2021) Differential regional responses in soluble monomeric alpha synuclein abundance following traumatic brain injury. Mol Neurobiol 58(1):362–374. https://doi.org/10.1007/s12035-020-02123-w
Kulbe JR, Hill RL, Singh IN, Wang JA, Hall ED (2017) Synaptic mitochondria sustain more damage than non-synaptic mitochondria after traumatic brain injury and are protected by cyclosporine A. J Neurotrauma 34(7):1291–1301. https://doi.org/10.1089/neu.2016.4628
Ondek K, Brevnova O, Jimenez-Ornelas C, Vergara A, Zwienenberg M, Gurkoff G (2020) A new model of repeat mTBI in adolescent rats. Exp Neurol 331:113360. https://doi.org/10.1016/j.expneurol.2020.113360
Smith DH, Okiyama K, Thomas MJ, Claussen B, McIntosh TK (1991) Evaluation of memory dysfunction following experimental brain injury using the Morris water maze. J Neurotrauma 8(4):259–269. https://doi.org/10.1089/neu.1991.8.259
Saatman KE, Contreras PC, Smith DH, Raghupathi R, McDermott KL, Fernandez SC, Sanderson KL, Voddi M et al (1997) Insulin-like growth factor-1 (IGF-1) improves both neurological motor and cognitive outcome following experimental brain injury. Exp Neurol 147(2):418–427. https://doi.org/10.1006/exnr.1997.6629
Saatman KE, Feeko KJ, Pape RL, Raghupathi R (2006) Differential behavioral and histopathological responses to graded cortical impact injury in mice. J Neurotrauma 23(8):1241–1253. https://doi.org/10.1089/neu.2006.23.1241
Kelly KM, Miller ER, Lepsveridze E, Kharlamov EA, McHedlishvili Z (2015) Posttraumatic seizures and epilepsy in adult rats after controlled cortical impact. Epilepsy Res 117:104–116. https://doi.org/10.1016/j.eplepsyres.2015.09.009
Bolkvadze T, Pitkanen A (2012) Development of post-traumatic epilepsy after controlled cortical impact and lateral fluid-percussion-induced brain injury in the mouse. J Neurotrauma 29(5):789–812. https://doi.org/10.1089/neu.2011.1954
Statler KD, Scheerlinck P, Pouliot W, Hamilton M, White HS, Dudek FE (2009) A potential model of pediatric posttraumatic epilepsy. Epilepsy Res 86(2–3):221–223. https://doi.org/10.1016/j.eplepsyres.2009.05.006
Feng G, Xiao F, Lu Y, Huang Z, Yuan J, Xiao Z, Xi Z, Wang X (2009) Down-regulation synaptic vesicle protein 2A in the anterior temporal neocortex of patients with intractable epilepsy. J Mol Neurosci 39(3):354–359. https://doi.org/10.1007/s12031-009-9288-2
van Vliet EA, Aronica E, Redeker S, Boer K, Gorter JA (2009) Decreased expression of synaptic vesicle protein 2A, the binding site for levetiracetam, during epileptogenesis and chronic epilepsy. Epilepsia 50(3):422–433. https://doi.org/10.1111/j.1528-1167.2008.01727.x
Contreras-Garcia IJ, Pichardo-Macias LA, Santana-Gomez CE, Sanchez-Huerta K, Ramirez-Hernandez R, Gomez-Gonzalez B, Rocha L, Mendoza Torreblanca JG (2018) Differential expression of synaptic vesicle protein 2A after status epilepticus and during epilepsy in a lithium-pilocarpine model. Epilepsy Behav 88:283–294. https://doi.org/10.1016/j.yebeh.2018.08.023
Hanaya R, Hosoyama H, Sugata S, Tokudome M, Hirano H, Tokimura H, Kurisu K, Serikawa T et al (2012) Low distribution of synaptic vesicle protein 2A and synaptotagimin-1 in the cerebral cortex and hippocampus of spontaneously epileptic rats exhibiting both tonic convulsion and absence seizure. Neuroscience 221:12–20. https://doi.org/10.1016/j.neuroscience.2012.06.058
Matveeva EA, Vanaman TC, Whiteheart SW, Slevin JT (2007) Asymmetric accumulation of hippocampal 7S SNARE complexes occurs regardless of kindling paradigm. Epilepsy Res 73(3):266–274. https://doi.org/10.1016/j.eplepsyres.2006.11.003
Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, Fuks B (2004) The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA 101(26):9861–9866. https://doi.org/10.1073/pnas.0308208101
Kaminski RM, Gillard M, Leclercq K, Hanon E, Lorent G, Dassesse D, Matagne A, Klitgaard H (2009) Proepileptic phenotype of SV2A-deficient mice is associated with reduced anticonvulsant efficacy of levetiracetam. Epilepsia 50(7):1729–1740. https://doi.org/10.1111/j.1528-1167.2009.02089.x
Browning M, Shear DA, Bramlett HM, Dixon CE, Mondello S, Schmid KE, Poloyac SM, Dietrich WD et al (2016) Levetiracetam treatment in traumatic brain injury: operation brain trauma therapy. J Neurotrauma 33(6):581–594. https://doi.org/10.1089/neu.2015.4131
Chen YH, Huang EY, Kuo TT, Hoffer BJ, Wu PJ, Ma HI, Tsai JJ, Chou YC et al (2016) Levetiracetam prophylaxis ameliorates seizure epileptogenesis after fluid percussion injury. Brain Res 1642:581–589. https://doi.org/10.1016/j.brainres.2016.04.013
Chen YH, Kuo TT, Yi-Kung Huang E, Hoffer BJ, Chou YC, Chiang YH, Ma HI, Miller JP (2018) Profound deficits in hippocampal synaptic plasticity after traumatic brain injury and seizure is ameliorated by prophylactic levetiracetam. Oncotarget 9(14):11515–11527. https://doi.org/10.18632/oncotarget.23923
Wang H, Gao J, Lassiter TF, McDonagh DL, Sheng H, Warner DS, Lynch JR, Laskowitz DT (2006) Levetiracetam is neuroprotective in murine models of closed head injury and subarachnoid hemorrhage. Neurocrit Care 5(1):71–78. https://doi.org/10.1385/NCC:5:1:71
Zou H, Brayer SW, Hurwitz M, Niyonkuru C, Fowler LE, Wagner AK (2013) Neuroprotective, neuroplastic, and neurobehavioral effects of daily treatment with levetiracetam in experimental traumatic brain injury. Neurorehabil Neural Repair 27(9):878–888. https://doi.org/10.1177/1545968313491007
Funding
This work was supported by the National Institutes of Health Grant 1R21NS111099 (SWC) and 5R01NS079061 (CED), The Chuck Noll Foundation (SWC), and the Walter L. Copeland Fund of The Pittsburgh Foundation (SWC).
Author information
Authors and Affiliations
Contributions
KMF, YL, JH, CED, and SWC completed the investigation; KMF and SWC completed data curation and analysis; KMF, CED, and SWC drafted, edited, and revised the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Research involving human participants: Not applicable. Research involving welfare of animal subjects: All experimental procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee in accordance with the guidelines established by the National Institutes of Health in the Guide for the Care and Use of Laboratory Animals.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fronczak, K.M., Li, Y., Henchir, J. et al. Reductions in Synaptic Vesicle Glycoprotein 2 Isoforms in the Cortex and Hippocampus in a Rat Model of Traumatic Brain Injury. Mol Neurobiol 58, 6006–6019 (2021). https://doi.org/10.1007/s12035-021-02534-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02534-3