Abstract
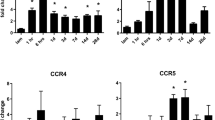
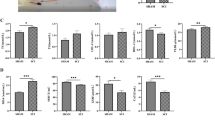
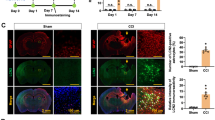
Lipocalin 2 (LCN2), an immunomodulator, regulates various cellular processes such as iron transport and defense against bacterial infection. Under pathological conditions, LCN2 promotes neuroinflammation via the recruitment and activation of immune cells and glia, particularly microglia and astrocytes. Although it seems to have a negative influence on the functional outcome in spinal cord injury (SCI), the extent of its involvement in SCI and the underlying mechanisms are not yet fully known. In this study, using a SCI contusion mouse model, we first investigated the expression pattern of Lcn2 in different parts of the CNS (spinal cord and brain) and in the liver and its concentration in blood serum. Interestingly, we could note a significant increase in LCN2 throughout the whole spinal cord, in the brain, liver, and blood serum. This demonstrates the diversity of its possible sites of action in SCI. Furthermore, genetic deficiency of Lcn2 (Lcn2−/−) significantly reduced certain aspects of gliosis in the SCI-mice. Taken together, our studies provide first valuable hints, suggesting that LCN2 is involved in the local and systemic effects post SCI, and might modulate the impairment of different peripheral organs after injury.




Similar content being viewed by others
Data Availability
The datasets generated and analyzed during this study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
Abbreviations
- ALDH1L1:
-
Aldehyde dehydrogenase 1 family member L1
- Bax:
-
Bcl-2-associated X protein
- Bcl2:
-
B-Cell lymphoma 2
- BSA:
-
Bovine serum albumin
- C3:
-
Complement component 3
- CD31:
-
Cluster of differentiation 31
- CD44:
-
Cluster of differentiation 44
- CNS:
-
Central nervous system
- EDTA:
-
Ethylenediaminetetraacetic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- FCS:
-
Fetal calf serum
- GFAP:
-
Glial fibrillary acidic protein
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- Hsp90:
-
Heat shock protein 90
- IBA1:
-
Ionized calcium binding adaptor molecule 1
- IgG:
-
Immunoglobulin G
- LCN2:
-
Lipocalin 2
- M-MLV:
-
Moloney murine leukemia virus
- NGAL:
-
Neutrophil gelatinase-associated lipocalin
- PBS:
-
Phosphate-buffered saline
- PCR:
-
Polymerase chain reaction
- PFA:
-
Paraformaldehyde
- PVDF:
-
Polyvinylidene difluoride
- RIPA:
-
Radioimmunoprecipitation assay
- RT:
-
Room temperature
- SC :
-
Spinal cord
- SCI:
-
Spinal cord injury
- SDS:
-
Sodium dodecyl sulfate
- SPHK1:
-
Sphingosine kinase 1
- TBS:
-
Tris-buffered saline
- WT:
-
Wild-type
References
Westgren N, Levi R (1998) Quality of life and traumatic spinal cord injury. Arch Phys Med Rehabil 79(11):1433–1439. https://doi.org/10.1016/s0003-9993(98)90240-4
Torregrossa F, Salli M, Grasso G (2020) Emerging therapeutic strategies for traumatic spinal cord injury. World Neurosurg 140:591–601. https://doi.org/10.1016/j.wneu.2020.03.199
Ahuja CS et al (2017) Traumatic spinal cord injury-repair and regeneration. Neurosurgery 80(3S):S9–S22. https://doi.org/10.1093/neuros/nyw080
Fleming JC et al (2006) The cellular inflammatory response in human spinal cords after injury. Brain 129(Pt 12):3249–3269. https://doi.org/10.1093/brain/awl296
Gattlen C et al (2016) Spinal cord T-cell infiltration in the rat spared nerve injury model: a time course study. Int J Mol Sci 17(3):352. https://doi.org/10.3390/ijms17030352
Faulkner JR et al (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24(9):2143–2155. https://doi.org/10.1523/JNEUROSCI.3547-03.2004
Sekhon LH, Fehlings MG (2001) Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 26(24 Suppl):S2-12. https://doi.org/10.1097/00007632-200112151-00002
Ducker TB, Assenmacher DR (1969) Microvascular response to experimental spinal cord trauma. Surg Forum 20:428–430
Guha A, Tator CH (1988) Acute cardiovascular effects of experimental spinal cord injury. J Trauma 28(4):481–490. https://doi.org/10.1097/00005373-198804000-00011
Pineau I et al (2010) Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL-1 receptor/MyD88-dependent fashion. Brain Behav Immun 24(4):540–553. https://doi.org/10.1016/j.bbi.2009.11.007
David S, Kroner A (2011) Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12(7):388–399. https://doi.org/10.1038/nrn3053
Taoka Y et al (1997) Role of neutrophils in spinal cord injury in the rat. Neuroscience 79(4):1177–1182. https://doi.org/10.1016/s0306-4522(97)00011-0
Popovich PG et al (1999) Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol 158(2):351–365. https://doi.org/10.1006/exnr.1999.7118
Cregg JM et al (2014) Functional regeneration beyond the glial scar. Exp Neurol 253:197–207. https://doi.org/10.1016/j.expneurol.2013.12.024
Kroner AJ (2019) Rosas Almanza, Role of microglia in spinal cord injury. Neurosci Lett 709:134370. https://doi.org/10.1016/j.neulet.2019.134370
Haydon PG, Carmignoto G (2006) Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86(3):1009–1031. https://doi.org/10.1152/physrev.00049.2005
Bush TG et al (1999) Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23(2):297–308. https://doi.org/10.1016/s0896-6273(00)80781-3
Firkins SS, Bates CA, Stelzner DJ (1993) Corticospinal tract plasticity and astroglial reactivity after cervical spinal injury in the postnatal rat. Exp Neurol 120(1):1–15. https://doi.org/10.1006/exnr.1993.1036
Davies SJ et al (1997) Regeneration of adult axons in white matter tracts of the central nervous system. Nature 390(6661):680–683. https://doi.org/10.1038/37776
Okada S et al (2006) Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med 12(7):829–834. https://doi.org/10.1038/nm1425
Bellver-Landete V et al (2019) Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat Commun 10(1):518. https://doi.org/10.1038/s41467-019-08446-0
Zamanian JL et al (2012) Genomic analysis of reactive astrogliosis. J Neurosci 32(18):6391–6410. https://doi.org/10.1523/JNEUROSCI.6221-11.2012
Brambilla R et al (2005) Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med 202(1):145–156. https://doi.org/10.1084/jem.20041918
Liddelow SA et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541(7638):481–487. https://doi.org/10.1038/nature21029
Jang E et al (2013) Phenotypic polarization of activated astrocytes: the critical role of lipocalin-2 in the classical inflammatory activation of astrocytes. J Immunol 191(10):5204–5219. https://doi.org/10.4049/jimmunol.1301637
Rathore KI et al (2011) Lipocalin 2 plays an immunomodulatory role and has detrimental effects after spinal cord injury. J Neurosci 31(38):13412–13419. https://doi.org/10.1523/jneurosci.0116-11.2011
Flo TH et al (2004) Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432(7019):917–921. https://doi.org/10.1038/nature03104
Al Nimer F et al (2016) Lipocalin-2 is increased in progressive multiple sclerosis and inhibits remyelination. Neurol Neuroimmunol Neuroinflamm 3(1):e191. https://doi.org/10.1212/NXI.0000000000000191
Ni W et al (2015) Role of lipocalin-2 in brain injury after intracerebral hemorrhage. J Cereb Blood Flow Metab 35(9):1454–1461. https://doi.org/10.1038/jcbfm.2015.52
Ranjbar Taklimie F et al (2019) Hypoxia induces astrocyte-derived lipocalin-2 in ischemic stroke. Int J Mol Sci 20(6).https://doi.org/10.3390/ijms20061271
Moschen AR et al (2017) Lipocalin-2: a master mediator of intestinal and metabolic inflammation. Trends Endocrinol Metab 28(5):388–397. https://doi.org/10.1016/j.tem.2017.01.003
Yang J et al (2002) An iron delivery pathway mediated by a lipocalin. Mol Cell 10(5):1045–1056. https://doi.org/10.1016/s1097-2765(02)00710-4
Bi F et al (2013) Reactive astrocytes secrete lcn2 to promote neuron death. Proc Natl Acad Sci U S A 110(10):4069–4074. https://doi.org/10.1073/pnas.1218497110
Jang E et al (2013) Secreted protein lipocalin-2 promotes microglial M1 polarization. FASEB J 27(3):1176–1190. https://doi.org/10.1096/fj.12-222257
Wu J et al (2014) Spinal cord injury causes brain inflammation associated with cognitive and affective changes: role of cell cycle pathways. J Neurosci 34(33):10989–11006. https://doi.org/10.1523/JNEUROSCI.5110-13.2014
Davidoff G et al (1985) Cognitive dysfunction and mild closed head injury in traumatic spinal cord injury. Arch Phys Med Rehabil 66(8):489–491
Davidoff GN, Roth EJ, Richards JS (1992) Cognitive deficits in spinal cord injury: epidemiology and outcome. Arch Phys Med Rehabil 73(3):275–284
Campbell SJ et al (2005) Central nervous system injury triggers hepatic CC and CXC chemokine expression that is associated with leukocyte mobilization and recruitment to both the central nervous system and the liver. Am J Pathol 166(5):1487–1497. https://doi.org/10.1016/S0002-9440(10)62365-6
Cheng RD et al (2020) Spinal cord injury causes insulin resistance associated with PI3K signaling pathway in hypothalamus. Neurochem Int 140:104839. https://doi.org/10.1016/j.neuint.2020.104839
Berger T et al (2006) Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci U S A 103(6):1834–1839. https://doi.org/10.1073/pnas.0510847103
Gasterich N et al (2021) Inflammatory responses of astrocytes are independent from lipocalin 2. J Mol Neurosci 71(5):933–942. https://doi.org/10.1007/s12031-020-01712-7
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12(1):1–21. https://doi.org/10.1089/neu.1995.12.1
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Neal M et al (2018) Prokineticin-2 promotes chemotaxis and alternative A2 reactivity of astrocytes. Glia 66(10):2137–2157. https://doi.org/10.1002/glia.23467
Dekens DW et al (2017) Neutrophil gelatinase-associated lipocalin and its receptors in Alzheimer’s disease (AD) brain regions: differential findings in AD with and without depression. J Alzheimers Dis 55(2):763–776. https://doi.org/10.3233/JAD-160330
Berard JL et al (2012) Lipocalin 2 is a novel immune mediator of experimental autoimmune encephalomyelitis pathogenesis and is modulated in multiple sclerosis. Glia 60(7):1145–1159. https://doi.org/10.1002/glia.22342
O’Shea TM, Burda JE, Sofroniew MV (2017) Cell biology of spinal cord injury and repair. J Clin Invest 127(9):3259–3270. https://doi.org/10.1172/JCI90608
Freeman KA et al (2015) Spinal cord protection via alpha-2 agonist-mediated increase in glial cell-line-derived neurotrophic factor. J Thorac Cardiovasc Surg 149(2):578–84 discussion 584-6.https://doi.org/10.1016/j.jtcvs.2014.10.037
Sofroniew MV (2014) Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist 20(2):160–172. https://doi.org/10.1177/1073858413504466
Siebert JR, Osterhout DJ (2011) The inhibitory effects of chondroitin sulfate proteoglycans on oligodendrocytes. J Neurochem 119(1):176–188. https://doi.org/10.1111/j.1471-4159.2011.07370.x
Tran AP, Warren PM, Silver J (2018) The biology of regeneration failure and success after spinal cord injury. Physiol Rev 98(2):881–917. https://doi.org/10.1152/physrev.00017.2017
Liu LR et al (2020) Interaction of microglia and astrocytes in the neurovascular unit. Front Immunol 11:1024. https://doi.org/10.3389/fimmu.2020.01024
Hyvarinen T et al (2019) Co-stimulation with IL-1beta and TNF-alpha induces an inflammatory reactive astrocyte phenotype with neurosupportive characteristics in a human pluripotent stem cell model system. Sci Rep 9(1):16944. https://doi.org/10.1038/s41598-019-53414-9
Lee S, Jha MK, Suk K (2015) Lipocalin-2 in the inflammatory activation of brain astrocytes. Crit Rev Immunol 35(1):77–84. https://doi.org/10.1615/critrevimmunol.2015012127
Mondal A et al (2020) Lipocalin 2 induces neuroinflammation and blood-brain barrier dysfunction through liver-brain axis in murine model of nonalcoholic steatohepatitis. J Neuroinflammation 17(1):201. https://doi.org/10.1186/s12974-020-01876-4
Rashad NM et al (2017) Lipocalin-2 expression and serum levels as early predictors of type 2 diabetes mellitus in obese women. IUBMB Life 69(2):88–97. https://doi.org/10.1002/iub.1594
Shashidharamurthy R et al (2013) Differential role of lipocalin 2 during immune complex-mediated acute and chronic inflammation in mice. Arthritis Rheum 65(4):1064–1073. https://doi.org/10.1002/art.37840
Lee S et al (2011) Lipocalin-2 Is a chemokine inducer in the central nervous system: role of chemokine ligand 10 (CXCL10) in lipocalin-2-induced cell migration. J Biol Chem 286(51):43855–43870. https://doi.org/10.1074/jbc.M111.299248
Bachman MA, Miller VL, Weiser JN (2009) Mucosal lipocalin 2 has pro-inflammatory and iron-sequestering effects in response to bacterial enterobactin. PLoS Pathog 5(10):e1000622. https://doi.org/10.1371/journal.ppat.1000622
Hamzic N, Blomqvist A, Nilsberth C (2013) Immune-induced expression of lipocalin-2 in brain endothelial cells: relationship with interleukin-6, cyclooxygenase-2 and the febrile response. J Neuroendocrinol 25(3):271–280. https://doi.org/10.1111/jne.12000
Noble LJ et al (2002) Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci 22(17):7526–7535
Johnson P et al (2000) A role for the cell adhesion molecule CD44 and sulfation in leukocyte-endothelial cell adhesion during an inflammatory response? Biochem Pharmacol 59(5):455–465. https://doi.org/10.1016/s0006-2952(99)00266-x
Lee S et al (2007) A dual role of lipocalin 2 in the apoptosis and deramification of activated microglia. J Immunol 179(5):3231–3241. https://doi.org/10.4049/jimmunol.179.5.3231
Ouali Alami N et al (2018) NF-kappaB activation in astrocytes drives a stage-specific beneficial neuroimmunological response in ALS. EMBO J 37(16). https://doi.org/10.15252/embj.201798697
Abella V et al (2015) The potential of lipocalin-2/NGAL as biomarker for inflammatory and metabolic diseases. Biomarkers 20(8):565–571. https://doi.org/10.3109/1354750X.2015.1123354
Gris D, Hamilton EF, Weaver LC (2008) The systemic inflammatory response after spinal cord injury damages lungs and kidneys. Exp Neurol 211(1):259–270. https://doi.org/10.1016/j.expneurol.2008.01.033
Zhang C et al (2018) Gut microbiota dysbiosis in male patients with chronic traumatic complete spinal cord injury. J Transl Med 16(1):353. https://doi.org/10.1186/s12967-018-1735-9
Campbell IL (2005) Cytokine-mediated inflammation, tumorigenesis, and disease-associated JAK/STAT/SOCS signaling circuits in the CNS. Brain Res Brain Res Rev 48(2):166–177. https://doi.org/10.1016/j.brainresrev.2004.12.006
Sundman MH et al (2017) The bidirectional gut-brain-microbiota axis as a potential nexus between traumatic brain injury, inflammation, and disease. Brain Behav Immun 66:31–44. https://doi.org/10.1016/j.bbi.2017.05.009
Zhang Y et al (2014) Lipocalin 2 expression and secretion is highly regulated by metabolic stress, cytokines, and nutrients in adipocytes. PLoS One 9(5):e96997. https://doi.org/10.1371/journal.pone.0096997
Mukhamedshina YO et al (2017) Systemic and local cytokine profile following spinal cord injury in rats: a multiplex analysis. Front Neurol 8:581. https://doi.org/10.3389/fneur.2017.00581
Molina L et al (2018) Hepatocyte-derived lipocalin 2 is a potential serum biomarker reflecting tumor burden in hepatoblastoma. Am J Pathol 188(8):1895–1909. https://doi.org/10.1016/j.ajpath.2018.05.006
Li H et al (2018) Hepatocytes and neutrophils cooperatively suppress bacterial infection by differentially regulating lipocalin-2 and neutrophil extracellular traps. Hepatology 68(4):1604–1620. https://doi.org/10.1002/hep.29919
Jayaraman A et al (2005) Identification of neutrophil gelatinase-associated lipocalin (NGAL) as a discriminatory marker of the hepatocyte-secreted protein response to IL-1beta: a proteomic analysis. Biotechnol Bioeng 91(4):502–515. https://doi.org/10.1002/bit.20535
Cowland JB et al (2003) Neutrophil gelatinase-associated lipocalin is up-regulated in human epithelial cells by IL-1 beta, but not by TNF-alpha. J Immunol 171(12):6630–6639. https://doi.org/10.4049/jimmunol.171.12.6630
Borkham-Kamphorst E, Drews F, Weiskirchen R (2011) Induction of lipocalin-2 expression in acute and chronic experimental liver injury moderated by pro-inflammatory cytokines interleukin-1beta through nuclear factor-kappaB activation. Liver Int 31(5):656–665. https://doi.org/10.1111/j.1478-3231.2011.02495.x
Fleming JC et al (2012) Remote inflammatory response in liver is dependent on the segmental level of spinal cord injury. J Trauma Acute Care Surg 72(5):1194–201 discussion 1202.https://doi.org/10.1097/TA.0b013e31824d68bd
Ferreira AC et al (2015) From the periphery to the brain: Lipocalin-2, a friend or foe? Prog Neurobiol 131:120–136. https://doi.org/10.1016/j.pneurobio.2015.06.005
Jin M et al (2014) Lipocalin-2 deficiency attenuates neuroinflammation and brain injury after transient middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab 34(8):1306–1314. https://doi.org/10.1038/jcbfm.2014.83
Borkham-Kamphorst E et al (2013) Protective effects of lipocalin-2 (LCN2) in acute liver injury suggest a novel function in liver homeostasis. Biochim Biophys Acta 1832(5):660–673. https://doi.org/10.1016/j.bbadis.2013.01.014
Asimakopoulou A et al (2014) Lipocalin-2 (LCN2) regulates PLIN5 expression and intracellular lipid droplet formation in the liver. Biochim Biophys Acta 1842(10):1513–1524. https://doi.org/10.1016/j.bbalip.2014.07.017
Wieser V et al (2016) Lipocalin 2 drives neutrophilic inflammation in alcoholic liver disease. J Hepatol 64(4):872–880. https://doi.org/10.1016/j.jhep.2015.11.037
Li T et al (2019) An update on reactive astrocytes in chronic pain. J Neuroinflammation 16(1):140. https://doi.org/10.1186/s12974-019-1524-2
Lee S et al (2009) Lipocalin-2 is an autocrine mediator of reactive astrocytosis. J Neurosci 29(1):234–249. https://doi.org/10.1523/JNEUROSCI.5273-08.2009
Zhao N et al (2019) Lipocalin-2 may produce damaging effect after cerebral ischemia by inducing astrocytes classical activation. J Neuroinflammation 16(1):168. https://doi.org/10.1186/s12974-019-1556-7
Franklin KBJ, Paxinos G (2019) Paxinos and Franklin’s the mouse brain in stereotaxic coordinates. Academic Press, Cambridge, United States
Acknowledgements
We thank Uta Zahn, Petra Ibold, and Helga Helten for their technical support. Furthermore, we would like to acknowledge Tak W Mak, (University of Toronto, Canada) for providing Lcn2 knockout animals.
Funding
This project was supported by an internal grant from RWTH Aachen University (A. Zendedel, START- 101/18).
Author information
Authors and Affiliations
Contributions
The study was conceptualized and designed by Adib Zendedel, Cordian Beyer, Tim Clarner, and Victoria Behrens. Material preparation, data collection, and analysis were performed by Victoria Behrens, Weiyi Zhao, Clara Voelz, Nina Müller, and Natalie Gasterich. The first draft of the manuscript was written by Victoria Behrens, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Adib Zendedel was responsible for the overall supervision of this study.
Corresponding author
Ethics declarations
Ethics Approval
All animals used in this study were acquired and cared for in accordance with the Federation of European Laboratory Associations (FELASA) recommendations. All experimental procedures and animal care were approved by the Review board for the Care of Animal Subjects of the district government (LANUV, Recklinghausen, North Rhine-Westphalia, Germany) or by the Review Board for the Care of Animal Subjects of the district government (ethic No. 962055, Tehran, Iran) respectively.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12035_2021_2530_MOESM1_ESM.pptx
Supplementary file1 Supplementary figure 1 Gene expression of astrogliosis markers Gfap (a), vimentin (b) and serpina3n (c) in central region of SC in WT mice (n=6; 72 h n=5). Ratio of Bax/Bcl2 in WT and Lcn2−/−mice in rostral (d) (n=5; Lcn2−/−control, 24 h n=4) and caudal (e) (n=5) region of SC. Data represent means ± SEM. ****p<0.0001, ***p<0.001, **p<0.01, *p<0.05 indicate control vs time point. Wherever a tendency towards a significant difference between WT and Lcn2−/−might be assumed, p values are given (PPTX 150 KB)
12035_2021_2530_MOESM2_ESM.pptx
Supplementary file2 Supplementary figure 2 IHC staining against astrogliosis marker ALDH1L1 of representative sections of the central SC from the groups control (a/d), 7 days WT (b/e) and 7 days Lcn2−/−(c/f) (PPTX 36552 KB)
Rights and permissions
About this article
Cite this article
Behrens, V., Voelz, C., Müller, N. et al. Lipocalin 2 as a Putative Modulator of Local Inflammatory Processes in the Spinal Cord and Component of Organ Cross talk After Spinal Cord Injury. Mol Neurobiol 58, 5907–5919 (2021). https://doi.org/10.1007/s12035-021-02530-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02530-7