Abstract
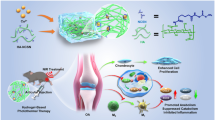
We investigate the anti-inflammatory effects of injectable hydrogel containing tauroursodeoxycholic acid (TUDCA) in a spinal cord injury (SCI) model. To this end, TUDCA-hydrogel (TC gel) is created by immersing the synthesized hydrogel in a TUDCA solution for 1 h. A mechanical SCI was imposed on rats, after which we injected the TC gel. After the SCI and injections, motor functions and lesions were significantly improved in the TC gel group compared with those in the saline group. The TC gel significantly decreased pro-inflammatory cytokine levels compared with the saline; TUDCA and glycol chitosan-oxidized hyaluronate were mixed at a ratio of 9:1 (CHA) gel independently. In addition, the TC gel significantly suppressed the phosphorylation of extracellular signal–regulated kinase (p-ERK) and c-Jun N-terminal kinase (p-JNK) in the mitogen-activated protein kinase (MAPK) pathway compared with the saline, TUDCA, and CHA gel independently. It also decreased tumor necrosis factor-α (TNF-α) and glial fibrillary acidic protein (GFAP), inflammatory marker, at the injured sites more than those in the saline, TUDCA, and CHA gel groups. In conclusion, the results of this study demonstrate the neuroinflammatory inhibition effects of TC gel in SCI and suggest that TC gel can be an alternative drug system for SCI cases.







Similar content being viewed by others
References
Li X, Curry EJ, Blais M, Ma R, Sungarian AS (2012) Intraspinal penetrating stab injury to the middle thoracic spinal cord with no neurologic deficit. Orthopedics 35(5):e770–e773. https://doi.org/10.3928/01477447-20120426-40
Bank M, Stein A, Sison C, Glazer A, Jassal N, McCarthy D, Shatzer M, Hahn B, Chugh R, Davies P, Bloom O (2015) Elevated circulating levels of the pro-inflammatory cytokine macrophage migration inhibitory factor in individuals with acute spinal cord injury. Arch Phys Med Rehabil 96 (4):633–644. https://doi.org/10.1016/j.apmr.2014.10.021
Khalatbary AR, Ahmadvand H (2011) Anti-inflammatory effect of the epigallocatechin gallate following spinal cord trauma in rat. Iran Biomed J 15(1–2):31–37
Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32(12):638–647. https://doi.org/10.1016/j.tins.2009.08.002
Tysseling-Mattiace VM, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, Stupp SI, Kessler JA (2008) Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci 28(14):3814–3823. https://doi.org/10.1523/JNEUROSCI.0143-08.2008
Bracken MB, Collins WF, Freeman DF, Shepard MJ, Wagner FW, Silten RM, Hellenbrand KG, Ransohoff J et al (1984) Efficacy of methylprednisolone in acute spinal cord injury. JAMA 251(1):45–52
Bartholdi D, Schwab ME (1995) Methylprednisolone inhibits early inflammatory processes but not ischemic cell death after experimental spinal cord lesion in the rat. Brain Res 672(1–2):177–186
Gerndt S, Rodriguez J, Pawlik J, Taheri P, Wahl W, Micheals A, Papadopoulos S (1997) Consequences of high-dose steroid therapy for acute spinal cord injury. J Trauma Acute Care Surg 42(2):279–284
Qian T, Guo X, Levi AD, Vanni S, Shebert R, Sipski M (2005) High-dose methylprednisolone may cause myopathy in acute spinal cord injury patients. Spinal Cord 43(4):199–203
Boatright JH, Nickerson JM, Moring AG, Pardue MT (2009) Bile acids in treatment of ocular disease. J Ocul Biol Dis Infor 2(3):149–159. https://doi.org/10.1007/s12177-009-9030-x
Bentayeb K, Batlle R, Sanchez C, Nerin C, Domeno C (2008) Determination of bile acids in human serum by on-line restricted access material-ultra high-performance liquid chromatography-mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 869(1–2):1–8. https://doi.org/10.1016/j.jchromb.2008.04.045
Van den Bossche L, Hindryckx P, Devisscher L, Devriese S, Van Welden S, Holvoet T, Vilchez-Vargas R, Vital M et al (2017) Ursodeoxycholic acid and its taurine- or glycine-conjugated species reduce colitogenic dysbiosis and equally suppress experimental colitis in mice. Appl Environ Microbiol 83(7). https://doi.org/10.1128/AEM.02766-16
Malo A, Krüger B, Seyhun E, Schäfer C, Hoffmann R-T, Göke B, Kubisch CH (2010) Tauroursodeoxycholic acid reduces endoplasmic reticulum stress, trypsin activation, and acinar cell apoptosis while increasing secretion in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol 299(4):G877–G886
Cho JG, Lee JH, Hong SH, Lee HN, Kim CM, Kim SY, Yoon KJ, Oh BJ et al (2015) Tauroursodeoxycholic acid, a bile acid, promotes blood vessel repair by recruiting vasculogenic progenitor cells. Stem Cells 33(3):792–805. https://doi.org/10.1002/stem.1901
Kim SJ, Ko WK, Jo MJ, Arai Y, Choi H, Kumar H, Han IB, Sohn S (2018) Anti-inflammatory effect of tauroursodeoxycholic acid in RAW 264.7 macrophages, bone marrow-derived macrophages, BV2 microglial cells, and spinal cord injury. Sci Rep 8(1):3176. https://doi.org/10.1038/s41598-018-21621-5
Kim SJ, Ko W-K, Heo DN, Lee SJ, Lee D, Heo M, Han I-B, Kwon IK et al (2019) Anti-neuroinflammatory gold nanocomplex loading ursodeoxycholic acid following spinal cord injury. Chem Eng J 375:122088
Miao L, Dong Y, Zhou FB, Chang YL, Suo ZG, Ding HQ (2018) Protective effect of tauroursodeoxycholic acid on the autophagy of nerve cells in rats with acute spinal cord injury. Eur Rev Med Pharmacol Sci 22(4):1133–1141. https://doi.org/10.26355/eurrev_201802_14402
Dong Y, Miao L, Hei L, Lin L, Ding H (2015) Neuroprotective effects and impact on caspase-12 expression of tauroursodeoxycholic acid after acute spinal cord injury in rats. Int J Clin Exp Pathol 8(12):15871–15878
Markman M (2003) Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol 4(5):277–283. https://doi.org/10.1016/s1470-2045(03)01074-x
Simmons RD, Willenborg DO (1990) Direct injection of cytokines into the spinal cord causes autoimmune encephalomyelitis-like inflammation. J Neurol Sci 100(1–2):37–42
Khaing ZZ, Milman BD, Vanscoy JE, Seidlits SK, Grill RJ, Schmidt CE (2011) High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J Neural Eng 8(4):046033. https://doi.org/10.1088/1741-2560/8/4/046033
Austin JW, Kang CE, Baumann MD, DiDiodato L, Satkunendrarajah K, Wilson JR, Stanisz GJ, Shoichet MS et al (2012) The effects of intrathecal injection of a hyaluronan-based hydrogel on inflammation, scarring and neurobehavioural outcomes in a rat model of severe spinal cord injury associated with arachnoiditis. Biomaterials 33(18):4555–4564. https://doi.org/10.1016/j.biomaterials.2012.03.022
Park J, Lim E, Back S, Na H, Park Y, Sun K (2010) Nerve regeneration following spinal cord injury using matrix metalloproteinase-sensitive, hyaluronic acid-based biomimetic hydrogel scaffold containing brain-derived neurotrophic factor. J Biomed Mater Res A 93(3):1091–1099
Zhang XZ, Jo Lewis P, Chu CC (2005) Fabrication and characterization of a smart drug delivery system: microsphere in hydrogel. Biomaterials 26(16):3299–3309. https://doi.org/10.1016/j.biomaterials.2004.08.024
Ko JA, Park HJ, Hwang SJ, Park JB, Lee JS (2002) Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int J Pharm 249(1–2):165–174. https://doi.org/10.1016/s0378-5173(02)00487-8
Kortesuo P, Ahola M, Kangas M, Jokinen M, Leino T, Vuorilehto L, Laakso S, Kiesvaara J et al (2002) Effect of synthesis parameters of the sol-gel-processed spray-dried silica gel microparticles on the release rate of dexmedetomidine. Biomaterials 23(13):2795–2801. https://doi.org/10.1016/s0142-9612(02)00016-9
Bhattarai N, Gunn J, Zhang M (2010) Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev 62(1):83–99. https://doi.org/10.1016/j.addr.2009.07.019
Ko WK, Kim SJ, Jo MJ, Choi H, Lee D, Kwon IK, Lee SH, Han IB et al (2019) Ursodeoxycholic acid inhibits inflammatory responses and promotes functional recovery after spinal cord injury in rats. Mol Neurobiol 56(1):267–277. https://doi.org/10.1007/s12035-018-0994-z
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12(1):1–21. https://doi.org/10.1089/neu.1995.12.1
Li J, Peng Y, Bao F, Liu A (2018) Correlation between gene polymorphism of macrophage migration inhibitory factor and disease susceptibility: recognition, target and significance. Zhongguo Zuzhi Gongcheng Yanjiu 22:4574–4579
Liu P, Peng J, Han GH, Ding X, Wei S, Gao G, Huang K, Chang F et al (2019) Role of macrophages in peripheral nerve injury and repair. Neural Regen Res 14(8):1335–1342. https://doi.org/10.4103/1673-5374.253510
Plemel JR, Wee Yong V, Stirling DP (2014) Immune modulatory therapies for spinal cord injury--past, present and future. Exp Neurol 258:91–104. https://doi.org/10.1016/j.expneurol.2014.01.025
Li M, Xu J, Mei X, Chi G, Li L, Song Y, He X, Li Y (2019) Regulatory effects of dermal papillary pluripotent stem cells on polarization of macrophages from M1 to M2 phenotype in vitro. Transpl Immunol 52:57–67. https://doi.org/10.1016/j.trim.2018.11.003
Kim DY, Park H, Kim SW, Lee JW, Lee KY (2017) Injectable hydrogels prepared from partially oxidized hyaluronate and glycol chitosan for chondrocyte encapsulation. Carbohydr Polym 157:1281–1287. https://doi.org/10.1016/j.carbpol.2016.11.002
Nair S, Remya N, Remya S, Nair PD (2011) A biodegradable in situ injectable hydrogel based on chitosan and oxidized hyaluronic acid for tissue engineering applications. Carbohydr Polym 85(4):838–844
Tan H, Chu CR, Payne KA, Marra KG (2009) Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 30(13):2499–2506. https://doi.org/10.1016/j.biomaterials.2008.12.080
Suh JK, Matthew HW (2000) Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials 21(24):2589–2598. https://doi.org/10.1016/s0142-9612(00)00126-5
Mohan N, Mohanan PV, Sabareeswaran A, Nair P (2017) Chitosan-hyaluronic acid hydrogel for cartilage repair. Int J Biol Macromol 104(Pt B):1936–1945. https://doi.org/10.1016/j.ijbiomac.2017.03.142
Macaya D, Spector M (2012) Injectable hydrogel materials for spinal cord regeneration: a review. Biomed Mater 7(1):012001. https://doi.org/10.1088/1748-6041/7/1/012001
Keene CD, Rodrigues CM, Eich T, Linehan-Stieers C, Abt A, Kren BT, Steer CJ, Low WC (2001) A bile acid protects against motor and cognitive deficits and reduces striatal degeneration in the 3-nitropropionic acid model of Huntington’s disease. Exp Neurol 171(2):351–360
Ramalho RM, Borralho PM, Castro RE, Sola S, Steer CJ, Rodrigues CM (2006) Tauroursodeoxycholic acid modulates p53-mediated apoptosis in Alzheimer’s disease mutant neuroblastoma cells. J Neurochem 98(5):1610–1618. https://doi.org/10.1111/j.1471-4159.2006.04007.x
Rodrigues CM, Spellman SR, Sola S, Grande AW, Linehan-Stieers C, Low WC, Steer CJ (2002) Neuroprotection by a bile acid in an acute stroke model in the rat. J Cereb Blood Flow Metab 22(4):463–471. https://doi.org/10.1097/00004647-200204000-00010
Rodrigues CM, Sola S, Nan Z, Castro RE, Ribeiro PS, Low WC, Steer CJ (2003) Tauroursodeoxycholic acid reduces apoptosis and protects against neurological injury after acute hemorrhagic stroke in rats. Proc Natl Acad Sci U S A 100(10):6087–6092. https://doi.org/10.1073/pnas.1031632100
Mantopoulos D, Murakami Y, Comander J, Thanos A, Roh M, Miller JW, Vavvas DG (2011) Tauroursodeoxycholic acid (TUDCA) protects photoreceptors from cell death after experimental retinal detachment. PLoS One 6(9):e24245. https://doi.org/10.1371/journal.pone.0024245
Kim BJ, Arai Y, Choi B, Park S, Ahn J, Han I-B, Lee S-H (2018) Restoration of articular osteochondral defects in rat by a bi-layered hyaluronic acid hydrogel plug with TUDCA-PLGA microsphere. J Ind Eng Chem 61:295–303
Edwards JP, Zhang X, Frauwirth KA, Mosser DM (2006) Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol 80(6):1298–1307
DiSabato DJ, Quan N, Godbout JP (2016) Neuroinflammation: the devil is in the details. J Neurochem 139:136–153
Yang L, Blumbergs PC, Jones NR, Manavis J, Sarvestani GT, Ghabriel MN (2004) Early expression and cellular localization of proinflammatory cytokines interleukin-1β, interleukin-6, and tumor necrosis factor-α in human traumatic spinal cord injury. Spine 29(9):966–971
Yang L, Jones NR, Blumbergs PC, Van Den Heuvel C, Moore EJ, Manavis J, Sarvestani GT, Ghabriel MN (2005) Severity-dependent expression of pro-inflammatory cytokines in traumatic spinal cord injury in the rat. J Clin Neurosci 12(3):276–284
Orr MB, Gensel JC (2018) Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics 15(3):541–553. https://doi.org/10.1007/s13311-018-0631-6
Rudge JS, Silver J (1990) Inhibition of neurite outgrowth on astroglial scars in vitro. J Neurosci 10(11):3594–3603
Nazari-Robati M, Akbari M, Khaksari M, Mirzaee M (2019) Trehalose attenuates spinal cord injury through the regulation of oxidative stress, inflammation and GFAP expression in rats. J Spinal Cord Med 42(3):387–394
Esposito E, Genovese T, Caminiti R, Bramanti P, Meli R, Cuzzocrea S (2009) Melatonin reduces stress-activated/mitogen-activated protein kinases in spinal cord injury. J Pineal Res 46(1):79–86. https://doi.org/10.1111/j.1600-079X.2008.00633.x
Xu Z, Wang BR, Wang X, Kuang F, Duan XL, Jiao XY, Ju G (2006) ERK1/2 and p38 mitogen-activated protein kinase mediate iNOS-induced spinal neuron degeneration after acute traumatic spinal cord injury. Life Sci 79(20):1895–1905. https://doi.org/10.1016/j.lfs.2006.06.023
Ip YT, Davis RJ (1998) Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr Opin Cell Biol 10(2):205–219
Koistinaho M, Koistinaho J (2002) Role of p38 and p44/42 mitogen-activated protein kinases in microglia. Glia 40(2):175–183
Ko WK, Lee SH, Kim SJ, Jo MJ, Kumar H, Han IB, Sohn S (2017) Anti-inflammatory effects of ursodeoxycholic acid by lipopolysaccharide-stimulated inflammatory responses in RAW 264.7 macrophages. PLoS One 12(6):e0180673. https://doi.org/10.1371/journal.pone.0180673
Funding
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and future planning (NRF-2016M3A9E8941668).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 1122 kb)
Rights and permissions
About this article
Cite this article
Han, G.H., Kim, S.J., Ko, WK. et al. Injectable Hydrogel Containing Tauroursodeoxycholic Acid for Anti-neuroinflammatory Therapy After Spinal Cord Injury in Rats. Mol Neurobiol 57, 4007–4017 (2020). https://doi.org/10.1007/s12035-020-02010-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02010-4