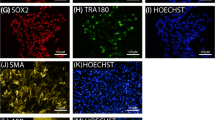
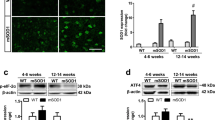
Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder with no cure. The reports showed the role of nearby astrocytes around the motor neurons as one among the causes of the disease. However, the exact mechanistic insights are not explored so far. Thus, in the present investigations, we employed the induced pluripotent stem cells (iPSCs) of Cu/Zn-SOD1L39R linked ALS patient to convert them into the motor neurons (MNs) and astrocytes. We report that the higher expression of stress granule (SG) marker protein G3BP1, and its co-localization with the mutated Cu/Zn-SOD1L39R protein in patient’s MNs and astrocytes are linked with AIF1-mediated upregulation of caspase 3/7 and hyper activated autophagy. We also observe the astrocyte-mediated non-cell autonomous neurotoxicity on MNs in ALS. The secretome of the patient’s iPSC-derived astrocytes exerts significant oxidative stress in MNs. The findings suggest the hyperactive status of autophagy in MNs, as witnessed by the co-distribution of LAMP1, P62 and LC3 I/II with the autolysosomes. Conversely, the secretome of normal astrocytes has shown neuroprotection in patient’s iPSC-derived MNs. The whole-cell patch-clamp assay confirms our findings at a physiological functional level in MNs. Perhaps for the first time, we are reporting that the MN degeneration in ALS triggered by the hyper-activation of autophagy and induced apoptosis in both cell-autonomous and non-cell autonomous conditions.








Similar content being viewed by others
References
Katz JS, Woolley SC, (2019) Amyotrophic lateral sclerosis, Physician's Field Guide to Neuropsychology, Springer, pp. 255–265.
Pansarasa O, Bordoni M, Diamanti L, Sproviero D, Gagliardi S, Cereda C (2018) SOD1 in amyotrophic lateral sclerosis: "ambivalent" behavior connected to the disease. Int J Mol Sci 19(5)
Lyon MS, Wosiski-Kuhn M, Gillespie R, Caress J, Milligan C (2019) Inflammation, immunity, and amyotrophic lateral sclerosis: I. Etiol Pathol Muscle Nerve 59(1):10–22
Chipika RH, Finegan E, Shing SLH, Hardiman O, Bede P (2019) Tracking a fast-moving disease: longitudinal markers, monitoring, and clinical trial endpoints in ALS. Front Neurol 10:229
Jaiswal MK (2019) Riluzole and edaravone: A tale of two amyotrophic lateral sclerosis drugs. Med Res Rev 39(2):733–748
Liu ZJ, Lin HX, Liu GL, Tao QQ, Ni W, Xiao BG, Wu ZY (2017) The investigation of genetic and clinical features in Chinese patients with juvenile amyotrophic lateral sclerosis. Clin Genet 92(3):267–273
Mattsson P, Lönnstedt I, Nygren I, Askmark H (2012) Physical fitness, but not muscle strength, is a risk factor for death in amyotrophic lateral sclerosis at an early age. J Neurol Neurosurg Psychiatry 83(4):390–394
Åberg M, Nyberg J, Robertson J, Kuhn G, Schiöler L, Nissbrandt H, Waern M, Torén K (2018) Risk factors in Swedish young men for amyotrophic lateral sclerosis in adulthood. J Neurol 265(3):460–470
Clement A, Nguyen M, Roberts E, Garcia M, Boillee S, Rule M, McMahon A, Doucette W et al (2003) Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science 302(5642):113–117
Marchetto MC, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH (2008) Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell 3(6):649–657
McAvoy KJ, (2019) Non-cell autonomous toxicity in Motor neuron disease: evidence and mechanisms of astrocyte-driven neurotoxicity in FUS-ALS, ETD collection for Thomas Jefferson University. AAI13425948
Djajadikerta A, Keshri S, Pavel M, Prestil R, Ryan L, Rubinsztein DC (2019) Autophagy induction as a therapeutic strategy for neurodegenerative diseases. J Mol Biol 432(8):2799–2821
Vijayalakshmi K, Alladi PA, Sathyaprabha T, Subramaniam JR, Nalini A, Raju T (2009) Cerebrospinal fluid from sporadic amyotrophic lateral sclerosis patients induces degeneration of a cultured motor neuron cell line. Brain Res 1263:122–133
Vijayalakshmi K, Alladi PA, Ghosh S, Prasanna V, Sagar B, Nalini A, Sathyaprabha T, Raju T (2011) Evidence of endoplasmic reticular stress in the spinal motor neurons exposed to CSF from sporadic amyotrophic lateral sclerosis patients. Neurobiol Dis 41(3):695–705
Sumitha R, Manjunatha VM, Sabitha RK, Alladi PA, Nalini A, Rao LT, Chandrasekhar Sagar BK, Steinbusch HWM et al (2018) Cerebrospinal fluid from patients with sporadic amyotrophic lateral sclerosis induces degeneration of motor neurons derived from human embryonic stem cells. Mol Neurobiol 56(2):1014–1034
Maimon R, Perlson E (2019) Muscle secretion of toxic factors, regulated by miR126-5p, facilitates motor neuron degeneration in amyotrophic lateral sclerosis. Neural Regen Res 14(6):969–970
Silverman JM, Christy D, Shyu CC, Moon KM, Fernando S, Gidden Z, Cowan CM, Ban Y et al (2019) CNS-derived extracellular vesicles from superoxide dismutase 1 (SOD1)(G93A) ALS mice originate from astrocytes and neurons and carry misfolded SOD1. J Biol Chem 294(10):3744–3759
Luscombe NM, Laskowski RA, Thornton JM (2001) Amino acid–base interactions: a three-dimensional analysis of protein–DNA interactions at an atomic level. Nucleic Acids Res 29(13):2860–2874
Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, Okochi H, Okuda A et al (2007) Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol 9(6):625–635
He S, Pant D, Schiffmacher A, Bischoff S, Melican D, Gavin W, Keefer C (2006) Developmental expression of pluripotency determining factors in caprine embryos: novel pattern of NANOG protein localization in the nucleolus. Mol Reprod Dev 73(12):1512–1522
Egawa N, Kitaoka S, Tsukita K, Naitoh M, Takahashi K, Yamamoto T, Adachi F, Kondo T et al (2012) Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci Transl Med 4(145):145ra104
Ma J, Guo R, Song Y, Zhang J, Feng B, Amponsah AE, Kong D, He J et al (2019) Generation and characterization of a human induced pluripotent stem cell (iPSC) line (HEBHMUi001-A) from a sporadic Parkinson's disease patient. Stem Cell Res 36:101417
Leferink P, Dooves S, Hillen A, Watanabe K, Jacobs G, Gasparotto L, Cornelissen-Steijger P, van der Knaap M, Heine V, (2019) Human and mouse iPSC-derived astrocyte subtypes reveal vulnerability in vanishing white matter, bioRxiv 523233.
Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP (2016) Sequential activation of microglia and astrocyte cytokine expression precedes increased iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia 64(2):300–316
Huai J, Zhang Z (2019) Structural properties and interaction partners of familial ALS-associated SOD1 mutants. Front Neurol 10:527
Nishitoh H, Kadowaki H, Nagai A, Maruyama T, Yokota T, Fukutomi H, Noguchi T, Matsuzawa A et al (2008) ALS-linked mutant SOD1 induces ER stress-and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev 22(11):1451–1464
Shah SZA, Hussain T, Zhao D, Yang L (2017) A central role for calcineurin in protein misfolding neurodegenerative diseases. Cell Mol Life Sci 74(6):1061–1074
Bonilla M, Nastase KK, Cunningham KW (2002) Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J 21(10):2343–2353
Baechler BL, Bloemberg D, Quadrilatero J (2019) Mitophagy regulates mitochondrial network signaling, oxidative stress, and apoptosis during myoblast differentiation. Autophagy 15(9):1606–1619 1–14
Del Grosso A, Angella L, Tonazzini I, Moscardini A, Giordano N, Caleo M, Rocchiccioli S, Cecchini M (2019) Dysregulated autophagy as a new aspect of the molecular pathogenesis of Krabbe disease. Neurobiol Dis 129:195–207
Stauffer W, Sheng H, Lim HN (2018) EzColocalization: An ImageJ plugin for visualizing and measuring colocalization in cells and organisms. Sci Rep 8(1):15764
Keller KE, Yang YF, Sun YY, Sykes R, Acott TS, Wirtz MK (2013) Ankyrin repeat and suppressor of cytokine signaling box containing protein-10 is associated with ubiquitin-mediated degradation pathways in trabecular meshwork cells. Mol Vis 19:1639–1655
Luzio JP, Hackmann Y, Dieckmann NM, Griffiths GM (2014) The biogenesis of lysosomes and lysosome-related organelles. Cold Spring Harb Perspect Biol 6(9):a016840
Basso M, Pozzi S, Tortarolo M, Fiordaliso F, Bisighini C, Pasetto L, Spaltro G, Lidonnici D et al (2013) Mutant copper-zinc superoxide dismutase (SOD1) induces protein secretion pathway alterations and exosome release in astrocytes implications for disease spreading and motor neuron pathology in amyotrophic lateral sclerosis. J Biol Chem 288(22):15699–15711
Choi SS, Lee HJ, Lim I, Satoh J-i, Kim SU (2014) Human astrocytes: secretome profiles of cytokines and chemokines. PLoS One 9(4):e92325
Chen L, Liu B (2017) Relationships between stress granules, oxidative stress, and neurodegenerative diseases. Oxidative Med Cell Longev 2017:1809592
Edens BM, Miller N, Ma YC (2016) Impaired autophagy and defective mitochondrial function: converging paths on the road to motor neuron degeneration. Front Cell Neurosci 10:44
Kang Y, Liu J, Song B, Feng X, Ou L, Wei L, Lai X, Shao L (2016) Potential links between cytoskeletal disturbances and electroneurophysiological dysfunctions induced in the central nervous system by inorganic nanoparticles. Cell Physiol Biochem 40(6):1487–1505
Effgen GB, Morrison B III (2017) Electrophysiological and pathological characterization of the period of heightened vulnerability to repetitive injury in an in vitro stretch model. J Neurotrauma 34(4):914–924
McComish SF, Caldwell MA (2018) Generation of defined neural populations from pluripotent stem cells. Philos Trans R Soc B: Biol Sci 373(1750):20170214
Bean B (2019) Context and complexity: how ionic conductances interact to control neuronal firing. Biophys J 116(3):9a–10a
Allodi I, Nijssen J, Benitez JA, Schweingruber C, Fuchs A, Bonvicini G, Cao M, Kiehn O, E. Hedlund, (2019) Modeling motor neuron resilience in ALS using stem cells, bioRxiv 399659.
Okita K, Yamakawa T, Matsumura Y, Sato Y, Amano N, Watanabe A, Goshima N, Yamanaka S (2013) An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells 31(3):458–466
Frati G, Luciani M, Meneghini V, De Cicco S, Stahlman M, Blomqvist M, Grossi S, Filocamo M et al (2018) Human iPSC-based models highlight defective glial and neuronal differentiation from neural progenitor cells in metachromatic leukodystrophy. Cell Death Dis 9(6):698
Meyer S, Worsdorfer P, Gunther K, Thier M, Edenhofer F, (2015) Derivation of adult human fibroblasts and their direct conversion into expandable neural progenitor cells, J Visual Exp (101):e52831.
Zhou L, Li P, Chen N, Dai LF, Gao K, Liu YN, Shen L, Wang JM et al (2019) Modeling vanishing white matter disease with patient-derived induced pluripotent stem cells reveals astrocytic dysfunction. CNS Neurosci Ther 25(6):759–771
Lee J, Hyeon SJ, Im H, Ryu H, Kim Y, Ryu H (2016) Astrocytes and microglia as non-cell autonomous players in the pathogenesis of ALS. Exp Neurobiol 25(5):233–240
Fernandes N, Eshleman N, Buchan JR, (2018), Stress granules and ALS: a case of causation or correlation?, RNA metabolism in neurodegenerative diseases, Springer pp. 173–212.
Zhang C-H, Wang J-X, Cai M-L, Shao R, Liu H, Zhao W-L (2019) The roles and mechanisms of G3BP1 in tumour promotion. J Drug Target 27(3):300–305
Chung CG, Lee H, Lee SB (2018) Mechanisms of protein toxicity in neurodegenerative diseases. Cell Mol Life Sci 75(17):3159–3180
Gal J, Kuang L, Barnett KR, Zhu BZ, Shissler SC, Korotkov KV, Hayward LJ, Kasarskis EJ et al (2016) ALS mutant SOD1 interacts with G3BP1 and affects stress granule dynamics. Acta Neuropathol 132(4):563–576
Monahan Z, Shewmaker F, Pandey UB (2016) Stress granules at the intersection of autophagy and ALS. Brain Res 1649(Pt B):189–200
Shao Q, Yang M, Liang C, Ma L, Zhang W, Jiang Z, Luo J, Lee JK, Liang C, Chen JF, (2019) C9orf72 and smcr8 mutant mice reveal MTORC1 activation due to impaired lysosomal degradation and exocytosis, Autophagy 1–16.
Oh YK, Shin KS, Kang SJ (2006) AIF translocates to the nucleus in the spinal motor neurons in a mouse model of ALS. Neurosci Lett 406(3):205–210
Myszczynska M, Ferraiuolo L (2016) New in vitro models to study amyotrophic lateral sclerosis. Brain Pathol 26(2):258–265
Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC (2008) Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell 3(6):637–648
Madill M, McDonagh K, Ma J, Vajda A, McLoughlin P, O'Brien T, Hardiman O, Shen S (2017) Amyotrophic lateral sclerosis patient iPSC-derived astrocytes impair autophagy via non-cell autonomous mechanisms. Molecular Brain 10(1):22
di Domenico A, Carola G, Calatayud C, Pons-Espinal M, Munoz JP, Richaud-Patin Y, Fernandez-Carasa I, Gut M et al (2019) Patient-specific iPSC-derived astrocytes contribute to non-cell-autonomous neurodegeneration in Parkinson's disease. Stem Cell Reports 12(2):213–229
Beers J, Gulbranson DR, George N, Siniscalchi LI, Jones J, Thomson JA, Chen G (2012) Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat Protoc 7(11):2029–2040
Krencik R, Zhang S-C (2011) Directed differentiation of functional astroglial subtypes from human pluripotent stem cells. Nat Protoc 6(11):1710–1717
Hu B-Y, Weick JP, Yu J, Ma L-X, Zhang X-Q, Thomson JA, Zhang S-C (2010) Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci 107(9):4335–4340
Shi Y, Kirwan P, Livesey FJ (2012) Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc 7(10):1836–1846
Acknowledgements
Authors are thankful to Dr. Patricia S. Kuriki for preparing lenti-viral particles and flowcytometric estimation at IBUSP, Brazil, and Mr. Puneet Khare for flowcytometric experiments at CSIR-IITR, Lucknow India. The technical assistance of Mr. Waldir Caldeira, IBUSP, Brazil, is acknowledged for operation of confocal microscopy. We thank to Dr. Manisha Mishra, CSIR-IITR, India, for helping in analysis of sequencing data and Dr. Naila Lourenço, IBUSP, Brazil, for assisting in the analysis of MLPA data. We also thank Mr. Thiago Giove Mitsugi, IBUSP, Brazil, for his technical support. The financial support from Department of Biotechnology, Ministry of Science & Technology, Government of India and CNPq-Brazil (Proc. No. 405243/2015-4) through Indo-Brazil project No. DBT/IC-2/Indo-Brazil/2016-19/02 are acknowledged. This study was also partly funded by FAPESP-CEPID (2013/08028-1), CNPq (307611/2018-3), INCT-CETGEN (573633/2008-8), and FINEP-CTC (0108057900). H.U. acknowledges grant support by the São Paulo Research Foundation (FAPESP project number.2012/50880-4). A.C. was supported by a postdoctoral fellowship from FAPESP (project number 2013/02293). OKO was a visiting scholar at HCCTD-HIAE.
Author information
Authors and Affiliations
Contributions
C.S.R. and A.B.P. designed the study. C.S.R. and V.K. performed the experiments on differentiating human iPSCs into motor neurons and astrocytes. M.Z. provided ALS patient’s fibroblasts and their clinical information. D.O. designed the experiments on iPSC reprogramming of the ALS patient’s fibroblasts. A.C. designed and performed the experiments on electrophysiology under the supervision of H.U., O.K.O. supervised the research activity and experiments at IBUSP, Brazil. C.S.R., V.K.K. and A.B.P. analysed the data.
Corresponding author
Ethics declarations
Conflict of Interest
All the authors have gone through the contents of the manuscript and given their consent to publish. There is no conflict of interest among the authors of this manuscript for scientific contents, financial aspects and otherwise.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rajpurohit, C.S., Kumar, V., Cheffer, A. et al. Mechanistic Insights of Astrocyte-Mediated Hyperactive Autophagy and Loss of Motor Neuron Function in SOD1L39R Linked Amyotrophic Lateral Sclerosis. Mol Neurobiol 57, 4117–4133 (2020). https://doi.org/10.1007/s12035-020-02006-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02006-0