Abstract
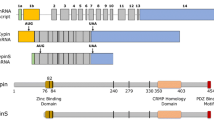
CRIPT, the cysteine-rich PDZ-binding protein, binds to the third PDZ domain of PSD-95 (postsynaptic density protein 95) family proteins and directly binds microtubules, linking PSD-95 family proteins to the neuronal cytoskeleton. Here, we show that overexpression of a full-length CRIPT leads to a modest decrease, and knockdown of CRIPT leads to an increase in dendritic branching in cultured rat hippocampal neurons. Overexpression of truncated CRIPT lacking the PDZ domain-binding motif, which does not bind to PSD-95, significantly decreases dendritic arborization. Conversely, overexpression of a full-length CRIPT significantly increases the number of immature and mature dendritic spines, and this effect is not observed when CRIPT∆PDZ is overexpressed. Competitive inhibition of CRIPT binding to the third PDZ domain of PSD-95 with PDZ3-binding peptides resulted in differential effects on dendritic arborization based on the origin of respective peptide sequence. These results highlight multifunctional roles of CRIPT during development and underscore the significance of the interaction between CRIPT and the third PDZ domain of PSD-95.






Similar content being viewed by others
References
Eilers J, Konnerth A (1997) Dendritic signal integration. Curr Opin Neurobiol 7(3):385–390. https://doi.org/10.1016/S0959-4388(97)80067-0
Hausser M, Spruston N, Stuart GJ (2000) Diversity and dynamics of dendritic signaling. Science (New York, NY) 290(5492):739–744
Vetter P, Roth A, Hausser M (2001) Propagation of action potentials in dendrites depends on dendritic morphology. J Neurophysiol 85(2):926–937. https://doi.org/10.1152/jn.2001.85.2.926
Kulkarni VA, Firestein BL (2012) The dendritic tree and brain disorders. Mol Cell Neurosci 50(1):10–20. https://doi.org/10.1016/j.mcn.2012.03.005
Kaufmann WE, Moser HW (2000) Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex (New York, NY : 1991) 10(10):981–991
Stephan KE, Baldeweg T, Friston KJ (2006) Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry 59(10):929–939. https://doi.org/10.1016/j.biopsych.2005.10.005
Calabresi P, Picconi B, Parnetti L, Di Filippo M (2006) A convergent model for cognitive dysfunctions in Parkinson's disease: the critical dopamine-acetylcholine synaptic balance. Lancet Neurol 5(11):974–983. https://doi.org/10.1016/s1474-4422(06)70600-7
Blanpied TA, Ehlers MD (2004) Microanatomy of dendritic spines: emerging principles of synaptic pathology in psychiatric and neurological disease. Biol Psychiatry 55(12):1121–1127. https://doi.org/10.1016/j.biopsych.2003.10.006
Südhof TC (2008) Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455(7215):903–911. https://doi.org/10.1038/nature07456
Gallo G (2011) The cytoskeletal and signaling mechanisms of axon collateral branching. Dev Neurobiol 71(3):201–220. https://doi.org/10.1002/dneu.20852
Stiess M, Bradke F (2011) Neuronal polarization: the cytoskeleton leads the way. Dev Neurobiol 71(6):430–444. https://doi.org/10.1002/dneu.20849
Jan YN, Jan LY (2010) Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci 11(5):316–328. https://doi.org/10.1038/nrn2836
Conde C, Cáceres A (2009) Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci 10(5):319–332. https://doi.org/10.1038/nrn2631
Parrish JZ, Emoto K, Kim MD, Jan YN (2007) Mechanisms that regulate establishment, maintenance, and remodeling of dendritic fields. Annu Rev Neurosci 30:399–423. https://doi.org/10.1146/annurev.neuro.29.051605.112907
Georges PC, Hadzimichalis NM, Sweet ES, Firestein BL (2008) The yin-yang of dendrite morphology: unity of actin and microtubules. Mol Neurobiol 38(3):270–284. https://doi.org/10.1007/s12035-008-8046-8
Sainath R, Gallo G (2015) Cytoskeletal and signaling mechanisms of neurite formation. Cell Tissue Res 359(1):267–278. https://doi.org/10.1007/s00441-014-1955-0
Kim E, Sheng M (2004) PDZ domain proteins of synapses. Nat Rev Neurosci 5(10):771–781. https://doi.org/10.1038/nrn1517
El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS (2000) PSD-95 involvement in maturation of excitatory synapses. Science (New York, NY) 290(5495):1364–1368
Kilinc D (2018) The emerging role of mechanics in synapse formation and plasticity. Front Cell Neurosci 12:483. https://doi.org/10.3389/fncel.2018.00483
Charych EI, Akum BF, Goldberg JS, Jornsten RJ, Rongo C, Zheng JQ, Firestein BL (2006) Activity-independent regulation of dendrite patterning by postsynaptic density protein PSD-95. J Neurosci 26(40):10164–10176. https://doi.org/10.1523/JNEUROSCI.2379-06.2006
Sweet ES, Previtera ML, Fernandez JR, Charych EI, Tseng CY, Kwon M, Starovoytov V, Zheng JQ et al (2011) PSD-95 alters microtubule dynamics via an association with EB3. J Neurosci 31(3):1038–1047. https://doi.org/10.1523/jneurosci.1205-10.2011
Yanai H, Satoh K, Matsumine A, Akiyama T (2000) The colorectal tumour suppressor APC is present in the NMDA-receptor-PSD-95 complex in the brain. Genes Cells : devoted to molecular & cellular mechanisms 5(10):815–822
Munemitsu S, Souza B, Muller O, Albert I, Rubinfeld B, Polakis P (1994) The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res 54(14):3676–3681
Takamori N, Shimomura A, Senda T (2006) Microtubule-bundling activity of APC is stimulated by interaction with PSD-95. Neurosci Lett 403(1–2):68–72. https://doi.org/10.1016/j.neulet.2006.04.045
Niethammer M, Valtschanoff JG, Kapoor TM, Allison DW, Weinberg RJ, Craig AM, Sheng M (1998) CRIPT, a Novel Postsynaptic Protein that Binds to the Third PDZ Domain of PSD-95/SAP90. Neuron 20(4):693–707. https://doi.org/10.1016/s0896-6273(00)81009-0
Passafaro M, Sala C, Niethammer M, Sheng M (1999) Microtubule binding by CRIPT and its potential role in the synaptic clustering of PSD-95. Nat Neurosci 2(12):1063–1069. https://doi.org/10.1038/15990
Bult CJ, Blake JA, Smith CL, Kadin JA, Richardson JE (2019) Mouse genome database (MGD) 2019. Nucleic Acids Res 47(D1):D801–d806. https://doi.org/10.1093/nar/gky1056
Shaheen R, Faqeih E, Ansari S, Abdel-Salam G, Al-Hassnan ZN, Al-Shidi T, Alomar R, Sogaty S et al (2014) Genomic analysis of primordial dwarfism reveals novel disease genes. Genome Res 24(2):291–299. https://doi.org/10.1101/gr.160572.113
Leduc MS, Niu Z, Bi W, Zhu W, Miloslavskaya I, Chiang T, Streff H, Seavitt JR et al (2016) CRIPT exonic deletion and a novel missense mutation in a female with short stature, dysmorphic features, microcephaly, and pigmentary abnormalities. Am J Med Genet A 170(8):2206–2211. https://doi.org/10.1002/ajmg.a.37780
Zhang L, Jablonski AM, Mojsilovic-Petrovic J, Ding H, Seeholzer S, Newton IP, Nathke I, Neve R et al (2017) SAP97 binding partner CRIPT promotes dendrite growth in vitro and in vivo. eNeuro 4(6):ENEURO.0175-17.2017. https://doi.org/10.1523/ENEURO.0175-17.2017
Kutzing MK, Langhammer CG, Luo V, Lakdawala H, Firestein BL (2010) Automated Sholl analysis of digitized neuronal morphology at multiple scales. J Vis Exp (45). doi:https://doi.org/10.3791/2354
Sweet ES, Langhammer CL, Kutzing MK, Firestein BL (2013) Semiautomated analysis of dendrite morphology in cell culture. Methods Mol Biol 1018:261–268. https://doi.org/10.1007/978-1-62703-444-9_24
Langhammer CG, Previtera ML, Sweet ES, Sran SS, Chen M, Firestein BL (2010) Automated Sholl analysis of digitized neuronal morphology at multiple scales: whole cell Sholl analysis versus Sholl analysis of arbor subregions. Cytometry A 77(12):1160–1168. https://doi.org/10.1002/cyto.a.20954
Saro D, Li T, Rupasinghe C, Paredes A, Caspers N, Spaller MR (2007) A thermodynamic ligand binding study of the third PDZ domain (PDZ3) from the mammalian neuronal protein PSD-95. Biochemistry 46(21):6340–6352. https://doi.org/10.1021/bi062088k
Patra CR, Rupasinghe CN, Dutta SK, Bhattacharya S, Wang E, Spaller MR, Mukhopadhyay D (2012) Chemically modified peptides targeting the PDZ domain of GIPC as a therapeutic approach for cancer. ACS Chem Biol 7(4):770–779. https://doi.org/10.1021/cb200536r
Dotti CG, Sullivan CA, Banker GA (1988) The establishment of polarity by hippocampal neurons in culture. J Neurosci 8(4):1454–1468. https://doi.org/10.1523/JNEUROSCI.08-04-01454.1988
Rademacher N, Kuropka B, Kunde S-A, Wahl MC, Freund C, Shoichet SA (2019) Intramolecular domain dynamics regulate synaptic MAGUK protein interactions. eLife 8:e41299. https://doi.org/10.7554/eLife.41299
Rademacher N, Kunde S-A, Vera S (2013) Synaptic MAGUK multimer formation is mediated by PDZ domains and promoted by ligand binding. Chem Biol 20(8):1044–1054. https://doi.org/10.1016/j.chembiol.2013.06.016
Zhu J, Shang Y, Zhang M (2016) Mechanistic basis of MAGUK-organized complexes in synaptic development and signalling. Nat Rev Neurosci 17(4):209–223. https://doi.org/10.1038/nrn.2016.18
Gerrow K, Romorini S, Nabi SM, Colicos MA, Sala C, El-Husseini A (2006) A preformed complex of postsynaptic proteins is involved in excitatory synapse development. Neuron 49(4):547–562. https://doi.org/10.1016/j.neuron.2006.01.015
Sigler A, Oh WC, Imig C, Altas B, Kawabe H, Cooper BH, Kwon H-B, Rhee J-S et al (2017) Formation and maintenance of functional spines in the absence of presynaptic glutamate release. Neuron 94(2):304–311.e304. https://doi.org/10.1016/j.neuron.2017.03.029
Cane M, Maco B, Knott G, Holtmaat A (2014) The relationship between PSD-95 clustering and spine stability in vivo. J Neurosci 34(6):2075–2086. https://doi.org/10.1523/JNEUROSCI.3353-13.2014
Zeng M, Shang Y, Araki Y, Guo T, Huganir RL, Zhang M (2016) Phase transition in postsynaptic densities underlies formation of synaptic complexes and synaptic plasticity. Cell 166(5):1163–1175.e1112. https://doi.org/10.1016/j.cell.2016.07.008
Akum BF, Chen M, Gunderson SI, Riefler GM, Scerri-Hansen MM, Firestein BL (2004) Cypin regulates dendrite patterning in hippocampal neurons by promoting microtubule assembly. Nat Neurosci 7(2):145–152. https://doi.org/10.1038/nn1179
Firestein BL, Brenman JE, Aoki C, Sanchez-Perez AM, El-Husseini AE, Bredt DS (1999) Cypin: a cytosolic regulator of PSD-95 postsynaptic targeting. Neuron 24(3):659–672
O'Neill KM, Donohue KE, Omelchenko A, Firestein BL (2018) The 3' UTRs of brain-derived neurotrophic factor transcripts differentially regulate the dendritic arbor. Front Cell Neurosci 12:60. https://doi.org/10.3389/fncel.2018.00060
Kwon M, Firestein BL (2013) DNA transfection: calcium phosphate method. Methods Mol Biol 1018:107–110. https://doi.org/10.1007/978-1-62703-444-9_10
Patel MV, Sewell E, Dickson S, Kim H, Meaney DF, Firestein BL (2019) A role for postsynaptic density 95 and its binding partners in models of traumatic brain injury. J Neurotrauma 36(13):2129–2138. https://doi.org/10.1089/neu.2018.6291
Funding
This work was funded by National Science Foundation grant IOS-1353724 and New Jersey Commission on Brain Injury Research grant #CBIR14IRG019 to B.L.F. A.O. was supported by National Institutes of Health Biotechnology Training Grant (T32 GM008339-20) and a predoctoral fellowship from the New Jersey Commission on Brain Injury Research #CBIR19FEL018. HM was supported by National Institutes of Health IRACDA (Institutional Research and Career Development Award) INSPIRE (IRACDA2K12GM093854-07A1). MS received funding from Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth.
Author information
Authors and Affiliations
Contributions
HM, MS, and BLF designed the experiments. HM, SD, GK, JR, HMC, ERM-M, and TW executed the experiments. HM, SD, GK, JR, and ERM-M performed the biochemical experiments and analysis of CRIPT overexpression and treatment of peptides on cultured neurons. HMC and TW synthesized the peptides. AO and BLF wrote the manuscript with input from HM and MS. AO analyzed all data. MS supervised peptide production and optimization, and BLF supervised the project.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Omelchenko, A., Menon, H., Donofrio, S.G. et al. Interaction Between CRIPT and PSD-95 Is Required for Proper Dendritic Arborization in Hippocampal Neurons. Mol Neurobiol 57, 2479–2493 (2020). https://doi.org/10.1007/s12035-020-01895-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01895-5