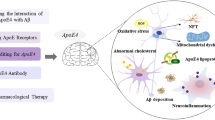
Abstract
The amyloid cascade hypothesis dealing with the senile plaques is until date thought to be one of the causative pathways leading to the pathophysiology of Alzheimer’s disease (AD). Though many aggregation inhibitors of misfolded amyloid beta (Aβ42) peptide have failed in clinical trials, there are some positive aspects of the designed therapeutic peptides for diseases involving proteinaceous aggregation. Here, we evaluated a smart design of side chain tripeptide (Leu-Val-Phe)-based polymeric inhibitor addressing the fundamental hydrophobic amino acid stretch “Lys-Leu-Val-Phe-Phe-Ala” (KLVFFA) of the Aβ42 peptide. The in vitro analyses performed through the thioflavin T (ThT) fluorescence assay, infrared spectroscopy, isothermal calorimetry, cytotoxicity experiments, and so on evinced a promising path towards the development of new age AD therapeutics targeting the inhibition of misfolded Aβ42 peptide fibrillization. The in silico simulations done contoured the mechanism of drug action of the present block copolymer as the competitive inhibition of aggregate-prone hydrophobic stretch of Aβ42.

The production of misfolded Aβ42 peptide from amyloid precursor protein initiates amyloidosis pathway which ends with the deposition of fibrils via the oligomerization and aggregation of Aβ42 monomers. The side chain tripeptide-based PEGylated polymer targets these Aβ42 monomers and oligomers inhibiting their aggregation. This block copolymer also binds and helps degrading the preformed fibrils of Aβ42.









Similar content being viewed by others
References
Khachaturian ZS (1985) Diagnosis of Alzheimer's disease. Arch Neurol 42:1097–1105
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP et al (2003) Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron 39:409–421
Reitz C, Brayne C, Mayeux R (2011) Epidemiology of Alzheimer disease. Nat Rev Neurol 7:137–152
Alzheimer’s Association (2018) Alzheimer’s disease facts and figures. Alzheimers Dement 14:367–429. https://doi.org/10.1016/j.jalz.2018.02.001
Khalsa DS, Perry G (2017) The four pillars of Alzheimer’s prevention. Cerebrum: The Dana Forum on Brain Science. 2017:cer-03-17. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5501038/. Accessed 1 Mar 2017
Prince M, Comas-Herrera A, Knapp M, Guerchet M, Karagiannidou M (2016) World Alzheimer report 2016: The global impact of dementia. Alzheimer's Disease International (ADI), London
Hardy JA, Higgins GA (1992) Alzheimer's disease: the amyloid cascade hypothesis. Science 256:184–185
Maccioni RB, Farías G, Morales I, Navarrete L (2010) The revitalized tau hypothesis on Alzheimer's disease. Arch Med Res 41:226–231
Swerdlow RH, Burns JM, Khan SM (2014) The Alzheimer's disease mitochondrial cascade hypothesis: progress and perspectives. Biochim Biophys Acta 1842:1219–1231
Chételat G, Villemagne VL, Bourgeat P (2010) Relationship between atrophy and β-amyloid deposition in Alzheimer disease. Ann Neurol 67:317–324
Greenough MA, Camakaris J, Bush AI (2013) Metal dyshomeostasis and oxidative stress in Alzheimer’s disease. Neurochem Int 62:540–555
Hane F (2013) Are amyloid fibrils molecular spandrels? FEBS Lett 587:3617–3619
Benilova I, Karran E, De Strooper B (2012) The toxic Aβ oligomer and Alzheimer's disease: an emperor in need of clothes. Nat Neurosci 15:349–357
Dutta S, Foley AR, Warner CJ (2017) Suppression of oligomer formation and formation of non-toxic fibrils upon addition of mirror-image Aβ42 to the natural l-enantiomer. Angew Chem Int Ed 56:11506–11510
Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid β-peptide. Nat Rev Mol Cell Biol 8:101–112
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297:353–356
Sullivan MG (2017) Alzheimer’s candidate drug Aducanumab moves to phase III. Caring for the Ages 18:18. https://doi.org/10.1016/j.carage.2017.02.015
Gao N, Sun H, Dong K, Ren J, Qu X (2015) Gold-nanoparticle-based multifunctional amyloid-β inhibitor against Alzheimer’s disease. Chem Eur J 21:829–835
Geng J, Li M, Ren J, Wang E, Qu X (2011) Polyoxometalates as inhibitors of the aggregation of amyloid β peptides associated with Alzheimer’s disease. Angew Chem Int Ed 123:4270–4274
Li M, Xu C, Ren J, Wang E, Qu X (2013) Photodegradation of β-sheet amyloid fibrils associated with Alzheimer's disease by using polyoxometalates as photocatalysts. Chem Commun 49:11394–11396
Wong HE, Qi W, Choi HM, Fernandez EJ, Kwon I (2011) A safe, blood-brain barrier permeable triphenylmethane dye inhibits amyloid-β neurotoxicity by generating nontoxic aggregates. ACS Chem Neurosci 2:645–657
Cohen SI, Arosio P, Presto J et al (2015) A molecular chaperone breaks the catalytic cycle that generates toxic Aβ oligomers. Nat Struct Mol Biol 22:207–213
Evans CG, Wisén S, Gestwicki JE (2006) Heat shock proteins 70 and 90 inhibit early stages of amyloid β-(1–42) aggregation in vitro. J Biol Chem 281:33182–33191
McKoy AF, Chen J, Schupbach T, Hecht MH (2012) A novel inhibitor of amyloid β (Aβ) peptide aggregation from high throughput screening to efficacy in an animal model of Alzheimer disease. J Biol Chem 287:38992–39000
Rezai-Zadeh K, Shytle D, Sun N, Mori T, Hou H, Jeanniton D, Ehrhart J, Townsend K et al (2005) Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci 25:8807–8814
Soto C, Sigurdsson EM, Morelli L, Kumar RA, Castaño EM, Frangione B (1998) β-Sheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis: implications for Alzheimer's therapy. Nat Med 4:822–826
Han X, Park J, Wu W, Malagon A, Wang L, Vargas E, Wikramanayake A, Houk KN et al (2017) A resorcinarene for inhibition of Aβ fibrillation. Chem Sci 8:2003–2009
Mukhopadhyay CD, Ruidas B, Chaudhury SS (2017) Role of curcumin in treatment of Alzheimer disease. Int J Neurorehabilitation 4:274
Skaat H, Chen R, Grinberg I, Margel S (2012) Engineered polymer nanoparticles containing hydrophobic dipeptide for inhibition of amyloid-β fibrillation. Biomacromolecules 13:2662–2670
Bachurin SO, Bovina EV, Ustyugov AA (2017) Drugs in clinical trials for Alzheimer's disease: the major trends. Med Res Rev 37:1186–1225
Cummings JL, Morstorf T, Zhong K (2014) Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther 6:37
Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M (2010) Alzheimer's disease: clinical trials and drug development. Lancet Neurol 9:702–716
Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, Mantua V, Mecocci P et al (2014) Clinical trials and late-stage drug development for Alzheimer's disease: an appraisal from 1984 to 2014. J Intern Med 275:251–283
Cheng YS, Chen ZT, Liao TY, Lin C et al (2017) An intranasally delivered peptide drug ameliorates cognitive decline in Alzheimer transgenic mice. EMBO Mol Med 9:703–715
Taylor M, Moore S, Mayes J, Parkin E, Beeg M, Canovi M, Gobbi M, Mann DMA et al (2010) Development of a proteolytically stable retro-inverso peptide inhibitor of β-amyloid oligomerization as a potential novel treatment for Alzheimer’s disease. Biochemistry 49:3261–3272
Som Chaudhury S, Das Mukhopadhyay C (2018) Functional amyloids: interrelationship with other amyloids and therapeutic assessment to treat neurodegenerative diseases. International Journal of Neuroscience 128:449–463
De Santis S, Chiaraluce R, Consalvi V et al (2017) PEGylated β-sheet breaker peptides as inhibitors of β-amyloid fibrillization. Chempluschem 82:241–250
Zhang C, Zheng X, Wan X, Shao X, Liu Q, Zhang Z, Zhang Q (2014) The potential use of H102 peptide-loaded dual-functional nanoparticles in the treatment of Alzheimer's disease. J Control Release 192:317–324
Zheng X, Shao X, Zhang C, Tan Y, Liu Q, Wan X, Zhang Q, Xu S et al (2015) Intranasal H102 peptide-loaded liposomes for brain delivery to treat Alzheimer’s disease. Pharm Res 32:3837–3849
Kumar S, Acharya R, Chatterji U, De P (2014) Controlled synthesis of β-sheet polymers based on side-chain amyloidogenic short peptide segments via RAFT polymerization. Polym Chem 5:6039–6050
Reinke AA, Gestwicki JE (2007) Structure–activity relationships of amyloid beta-aggregation inhibitors based on curcumin: influence of linker length and flexibility. Chem Biol Drug Des 70:206–215
Saleem S, Biswas SC (2017) Tribbles pseudokinase 3 induces both apoptosis and autophagy in amyloid-β-induced neuronal death. J Biol Chem 292:2571–2585
Lee KH, Shin BH, Shin KJ, Kim DJ, Yu J (2005) A hybrid molecule that prohibits amyloid fibrils and alleviates neuronal toxicity induced by β-amyloid (1–42). Biochem Biophys Res Commun 328:816–823
Ahmed M, Davis J, Aucoin D, Sato T, Ahuja S, Aimoto S, Elliott JI, van Nostrand WE et al (2010) Structural conversion of neurotoxic amyloid-β 1–42 oligomers to fibrils. Nat Struct Mol Biol 17:561–567
Dehle FC, Ecroyd H, Musgrave IF, Carver JA (2010) αB-Crystallin inhibits the cell toxicity associated with amyloid fibril formation by κ-casein and the amyloid-β peptide. Cell Stress Chaperones 15:1013–1026
Colvin MT, Silvers R, Ni QZ, Can TV, Sergeyev I, Rosay M, Donovan KJ, Michael B et al (2016) Atomic resolution structure of monomorphic Aβ42 amyloid fibrils. J Am Chem Soc 138:9663–9674
Pham JD, Spencer RK, Chen KH, Nowick JS (2014) A fibril-like assembly of oligomers of a peptide derived from β-amyloid. J Am Chem Soc 136:12682–12690
Crescenzi O, Tomaselli S, Guerrini R, Salvadori S, D'Ursi AM, Temussi PA, Picone D (2002) Solution structure of the Alzheimer amyloid β-peptide (1–42) in an apolar microenvironment: similarity with a virus fusion domain. Eur J Biochem 269:5642–5648
Colletier JP, Laganowsky A, Landau M, Zhao M, Soriaga AB, Goldschmidt L, Flot D, Cascio D et al (2011) Molecular basis for amyloid-β polymorphism. Proc Natl Acad Sci U S A 108:16938–16943
Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD et al (2005) Scalable molecular dynamics with NAMD. J Comput Chem 26:1781–1802
MacKerell AD Jr, Bashford D, Bellott ML et al (1998) All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B 102:3586–3616
Jorgensen WL, Madura JD (1983) Solvation and conformation of methanol in water. J Am Chem Soc 105:1407–1413
Vanommeslaeghe K, Hatcher E, Acharya C, Kundu S, Zhong S, Shim J, Darian E, Guvench O et al (2010) CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J Comput Chem 31:671–690
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
DeLano WL (2009) The PyMOL molecular graphics system 2009. DeLano Scientific, San Carlos
Sannigrahi A, Maity P, Karmakar S, Chattopadhyay K (2017) Interaction of KMP-11 with phospholipid membranes and its implications in leishmaniasis: effects of single tryptophan mutations and cholesterol. J Phys Chem B 121:1824–1834
Jameson LP, Smith NW, Dzyuba SV (2012) Dye-binding assays for evaluation of the effects of small molecule inhibitors on amyloid (Aβ) self-assembly. ACS Chem Neurosci 3:807–819
Knight JD, Miranker AD (2004) Phospholipid catalysis of diabetic amyloid assembly. J Mol Biol 341:1175–1187
Li J, Tian C, Yuan Y, Yang Z, Yin C, Jiang R, Song W, Li X et al (2015) A water-soluble conjugated polymer with pendant disulfide linkages to PEG chains: a highly efficient ratiometric probe with solubility-induced fluorescence conversion for thiol detection. Macromolecules 48:1017–1025
Adochitei A, Drochioiu G (2011) Rapid characterization of peptide secondary structure by FT-IR spectroscopy. Rev Roum Chim 56:783–791
Zandomeneghi G, Krebs MR, McCammon MG, Fändrich M (2004) FTIR reveals structural differences between native β-sheet proteins and amyloid fibrils. Protein Sci 13:3314–3321
Castelletto V, Ryumin P, Cramer R, Hamley IW, Taylor M, Allsop D, Reza M, Ruokolainen J et al (2017) Self-assembly and anti-amyloid cytotoxicity activity of amyloid beta peptide derivatives. Sci Rep 7:43637
Hubin E, Deroo S, Schierle GK, Kaminski C, Serpell L, Subramaniam V, van Nuland N, Broersen K et al (2015) Two distinct β-sheet structures in Italian-mutant amyloid-beta fibrils: a potential link to different clinical phenotypes. Cell Mol Life Sci 72:4899–4913
Sarkar-Banerjee S, Chowdhury S, Paul SS, Dutta D, Ghosh A, Chattopadhyay K (2016) The non-native helical intermediate state may accumulate at low pH in the folding and aggregation landscape of the intestinal fatty acid binding protein. Biochemistry 55:4457–4468
Amini Z, Fatemi MH, Rauk A (2016) Molecular dynamics studies of a β-sheet blocking peptide with the full-length amyloid beta peptide of Alzheimer’s disease. Can J Chem 94:833–841
Xu Y, Shen J, Luo X, Zhu W, Chen K, Ma J, Jiang H (2005) Conformational transition of amyloid β-peptide. Proc Natl Acad Sci U S A 102:5403–5407
Xie L, Luo Y, Wei G (2013) Aβ (16–22) peptides can assemble into ordered β-barrels and bilayer β-sheets, while substitution of phenylalanine 19 by tryptophan increases the population of disordered aggregates. J Phys Chem B 117:10149–10160
Zhang M, Chen J, Tian Z, Wang H (2017) Reply to the ‘Comment on “Magnetic-field-enabled resolution enhancement in super-resolution imaging”’ by Bergmann et al., Physical Chemistry Chemical Physics, 2017, 19. Phys Chem Chem Phys 19:4891–4892
Berhanu WM, Hansmann UH (2013) The stability of cylindrin β-barrel amyloid oligomer models—a molecular dynamics study. Proteins 81:1542–1555
Han X, Tian C, Gandra I, Eslava V, Galindres D, Vargas E, Leblanc R (2017) The investigation on Resorcinarenes towards either inhibiting or promoting insulin fibrillation. Chem Eur J 23:17903–17907
Simmons LK, May PC, Tomaselli KJ, Rydel RE, Fuson KS, Brigham EF, Wright S, Lieberburg I et al (1994) Secondary structure of amyloid beta peptide correlates with neurotoxic activity in vitro. Mol Pharmacol 45:373–379
Soto C, Castaño EM, Kumar RA, Beavis RC, Frangione B (1995) Fibrillogenesis of synthetic amyloid-β peptides is dependent on their initial secondary structure. Neurosci Lett 200:105–108
Jarvet J, Damberg P, Bodell K, Göran Eriksson LE, Gräslund A (2000) Reversible random coil to β-sheet transition and the early stage of aggregation of the Aβ (12–28) fragment from the Alzheimer peptide. J Am Chem Soc 122:4261–4268
Sureshbabu N, Kirubagaran R, Jayakumar R (2009) Surfactant-induced conformational transition of amyloid β-peptide. Eur Biophys J 38:355–367
Acknowledgements
We thank Mr. Kashinath Sahu at the Indian Institute of Science Education and Research (IISER), Kolkata, and Mr. Satyabrata Samaddar at the CSIR—Indian Institute of Chemical Biology (IICB), Kolkata, for the assistance with the FE-SEM and FT-IR work, respectively.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1479 kb).
Rights and permissions
About this article
Cite this article
Som Chaudhury, S., Sannigrahi, A., Nandi, M. et al. A Novel PEGylated Block Copolymer in New Age Therapeutics for Alzheimer’s Disease. Mol Neurobiol 56, 6551–6565 (2019). https://doi.org/10.1007/s12035-019-1542-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1542-1