Abstract
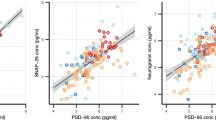
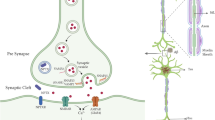
A loss of synaptic density and connectivity is observed in multiple brain regions of Alzheimer’s disease (AD) patients, resulting in a reduced expression of synaptic proteins such as SNAP-25 (synaptosomal-associated-protein-25). SNAP-25 alterations thus could be an index of the degree of synaptic degeneration in the central nervous system (CNS). We isolated from serum of both AD patients and healthy controls (HC) a population of neuron-derived exosomes (NDEs) and measured the concentrations of SNAP-25 contained in such NDEs. The levels of SNAP-25 carried by NDEs were reduced in AD patients (mean 459.05 ng/ml, SD 146.35 ng/ml) compared to HC (mean 686.42 ng/ml, SD 204.08 ng/ml) (p < 0.001). As a further confirmation of these results, ROC (receiver operating characteristic) analyses indicated that the level of SNAP-25 carried by NDEs has the power to discriminate between AD and HC (AUC = 0.826, sensitivity = 87.5%, specificity = 70.6%, p < 0.0001, cut-off value 587.07 ng/ml). Notably, a correlation between the levels of SNAP-25 carried by NDEs and levels and cognitive status measured by MMSE score (r = 0.465, 95% CI 0.11 to 0.714, p = 0.01) was detected. This is the first report of SNAP-25 measurement in serum. These data suggest that NDE-carried SNAP-25 could be an effective and accessible biomarker that reflects synapses integrity in the brain.




Similar content being viewed by others
References
Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, Brodaty H, Cedazo-Minguez A, Dubois B et al (2016) Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol 15(5):455–532. https://doi.org/10.1016/S1474-4422(16)00062-4
Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW Jr, Morris JC (2001) Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology 56(1):127–129
Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A et al (2008) Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science 321(5896):1686–1689. https://doi.org/10.1126/science.1162844
Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA et al (2008) Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 14(8):837–842. https://doi.org/10.1038/nm1782
Jahn R (1999) Recycling of synaptic vesicle membrane within nerve terminals. Brain Res Bull 50(5–6):313–314
Kvartsberg H, Duits FH, Ingelsson M, Andreasen N, Öhrfelt A, Andersson K, Brinkmalm G, Lannfelt L et al (2015) Cerebrospinal fluid levels of the synaptic protein neurogranin correlates with cognitive decline in prodromal Alzheimer’s disease. Alzheimers Dement 11(10):1180–1190. https://doi.org/10.1016/j.jalz.2014.10.009
Brinkmalm A, Brinkmalm G, Honer WG, Frölich L, Hausner L, Minthon L, Hansson O, Wallin A et al (2014) SNAP-25 is a promising novel cerebrospinal fluid biomarker for synapse degeneration in Alzheimer’s disease. Mol Neurodegener 9:53. https://doi.org/10.1186/1750-1326-9-53
Thompson PM, Rosenberger C, Holt S et al (1998) Measuring synaptosomal associated protein-25 kDa in human cerebral spinal fluid. J Psychiatr Res 32(5):297–300
Bereczki E, Bogstedt A, Höglund K, Tsitsi P, Brodin L, Ballard C, Svenningsson P, Aarsland D (2017) Synaptic proteins in CSF relate to Parkinson’s disease stage markers. NPJ Parkinsons Dis 3:7. https://doi.org/10.1038/s41531-017-0008-2
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr et al (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3):280–292. https://doi.org/10.1016/j.jalz.2011.03.003
Lashley T, Schott JM, Weston P, Murray CE, Wellington H, Keshavan A, et al (2018) Molecular biomarkers of Alzheimer’s disease: progress and prospects. Dis Model Mech 11(5). doi: https://doi.org/10.1242/dmm.031781.
Urbanelli L, Magini A, Buratta S, Brozzi A, Sagini K, Polchi A, Tancini B, Emiliani C (2013) Signaling pathways in exosomes biogenesis, secretion and fate. Genes (Basel) 4(2):152–170. https://doi.org/10.3390/genes4020152
Harding C, Heuser J, Stahl P (1983) Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 97(2):329–339
Bellingham SA, Guo BB, Coleman BM, Hill AF (2012) Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front Physiol 3:124. https://doi.org/10.3389/fphys.2012.00124
Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mäger I et al (2015) Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 4:26316. https://doi.org/10.3402/jev.v4.26316
Bala S, Csak T, Momen-Heravi F, Lippai D, Kodys K, Catalano D, Satishchandran A, Ambros V et al (2015) Biodistribution and function of extracellular miRNA-155 in mice. Sci Rep 5:10721. https://doi.org/10.1038/srep10721
Chen CC, Liu L, Ma F, Wong CW, Guo XE, Chacko JV, Farhoodi HP, Zhang SX et al (2016) Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell Mol Bioeng 9(4):509–529. https://doi.org/10.1007/s12195-016-0458-3
Noerholm M, Balaj L, Limperg T, Salehi A, Zhu LD, Hochberg FH, Breakefield XO, Carter BS et al (2012) RNA expression patterns in serum microvesicles from patients with glioblastoma multiforme and controls. BMC Cancer 12:22. https://doi.org/10.1186/1471-2407-12-22
Chen WW, Balaj L, Liau LM, Samuels ML, Kotsopoulos SK, Maguire CA, LoGuidice L, Soto H et al (2013) BEAMing and droplet digital PCR analysis of mutant IDH1 mRNA in glioma patient serum and cerebrospinal fluid extracellular vesicles. Mol Ther Nucleic Acids 2:e109. https://doi.org/10.1038/mtna.2013.28.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Service Task Force on Alzheimer’s disease. Neurology 34(7):939–944. https://doi.org/10.1212/WNL.34.7.939
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Association, Washington, DC
Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state. A practical method for grading the cognitive state of patients for the clinicians. J Psychiatr Res 2(3):189–198. https://doi.org/10.1016/0022-3956(75)90026-6
Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL (1982) A new clinical scale for staging of dementia. Br J Psychiatry 140:566–572. https://doi.org/10.1192/bjp.140.6.566
Helwa I, Cai J, Drewry MD, Zimmerman A, Dinkins MB, Khaled ML, Seremwe M, Dismuke WM et al (2017) A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. PLoS One 12(1):e0170628. https://doi.org/10.1371/journal.pone.0170628
Mustapic M, Eitan E, Werner JK Jr, Berkowitz ST, Lazaropoulos MP, Tran J, Goetzl EJ, Kapogiannis D (2017) Plasma extracellular vesicles enriched for neuronal origin: a potential window into brain pathologic processes. Front Neurosci 11:278. https://doi.org/10.3389/fnins.2017.00278
Fauré J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G et al (2006) Exosomes are released by cultured cortical neurones. Mol Cell Neurosci 31(4):642–648
Honer WG (2003) Pathology of presynaptic proteins in Alzheimer’s disease: more than simple loss of terminals. Neurobiol Aging 24(8):1047–1062
Goetzl EJ, Mustapic M, Kapogiannis D, Eitan E, Lobach IV, Goetzl L, Schwartz JB, Miller BL (2016) Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J 30(11):3853–3859
Goetzl EJ, Kapogiannis D, Schwartz JB, Lobach IV, Goetzl L, Abner EL, Jicha GA, Karydas AM et al (2016) Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J 30(12):4141–4148
Öhrfelt A, Brinkmalm A, Dumurgier J, Zetterberg H, Bouaziz-Amar E, Hugon J et al (2018) A novel ELISA for the measurement of cerebrospinal fluid SNAP-25 in patients with Alzheimer’s disease. Neuroscience. https://doi.org/10.1016/j.neuroscience
Zhang H, Therriault J, Kang MS, Ng KP, Pascoal TA, Rosa-Neto P et al (2018) Cerebrospinal fluid synaptosomal-associated protein 25 is a key player in synaptic degeneration in mild cognitive impairment and Alzheimer’s disease. Alzheimers Res Ther 10(1):80. https://doi.org/10.1186/s13195-018-0407-6
Wang S, Zhang J, Pan T, for Alzheimer’s Disease Neuroimaging Initiative (2018) APOE ε4 is associated with higher levels of CSF SNAP-25 in prodromal Alzheimer’s disease. Neurosci Lett 685:109–113. https://doi.org/10.1016/j.neulet.2018.08.029
Wakabayashi K, Honer WG, Masliah E (1994) Synapse alterations in the hippocampal-entorhinal formation in Alzheimer’s disease with and without Lewy body disease. Brain Res 667(1):24–32
Shimohama S, Kamiya S, Taniguchi T, Akagawa K, Kimura J (1997) Differential involvement of synaptic vesicle and presynaptic plasma membrane proteins in Alzheimer’s disease. Biochem Biophys Res Commun 236(2):239–242
Minger SL, Honer WG, Esiri MM, McDonald B, Keene J, Nicoll JA et al (2001) Synaptic pathology in prefrontal cortex is present only with severe dementia in Alzheimer disease. J Neuropathol Exp Neurol 60(10):929–936
Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, Carlson OD, Mustapic M et al (2015) Low neural exosomal levels of cellular survival factors in Alzheimer’s disease. Ann Clin Transl Neurol 2(7):769–773. https://doi.org/10.1002/acn3.211
Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB et al (2015) Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement 11(6):600–7.e1. https://doi.org/10.1016/j.jalz.2014.06.008
Kester MI, Teunissen CE, Crimmins DL, Herries EM, Ladenson JH, Scheltens P, van der Flier WM, Morris JC et al (2015) Neurogranin as a cerebrospinal fluid biomarker for synaptic loss in symptomatic Alzheimer disease. JAMA Neurol 72(11):1275–1280. https://doi.org/10.1001/jamaneurol.2015.1867
Wellington H, Paterson RW, Portelius E, Törnqvist U, Magdalinou N, Fox NC, Blennow K, Schott JM et al (2016) Increased CSF neurogranin concentration is specific to Alzheimer disease. Neurology 86(9):829–835. https://doi.org/10.1212/WNL.0000000000002423
Jahn R, Fasshauer D (2012) Molecular machines governing exocytosis of synaptic vesicles. Nature 490(7419):201–207. https://doi.org/10.1038/nature11320
Funding
This work was supported by Italian Ministry of Health (Ricerca Corrente 2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study conformed to the ethical principles of the Helsinki Declaration. The Ethical Committee of the Don C. Gnocchi Foundation IRCCS approved the study (Prot. N°10/2018/CE_FdG/SA); all the participants when possible, or patients’ legal guardians when it was not, gave informed consent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Agliardi, C., Guerini, F.R., Zanzottera, M. et al. SNAP-25 in Serum Is Carried by Exosomes of Neuronal Origin and Is a Potential Biomarker of Alzheimer’s Disease. Mol Neurobiol 56, 5792–5798 (2019). https://doi.org/10.1007/s12035-019-1501-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1501-x