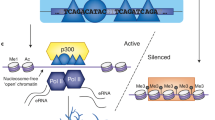
Abstract
A promoter can be regulated by various cis-acting elements so that delineation of the regulatory modes among them may help understand developmental, environmental and genetic mechanisms in gene activity. Here we report that the human dopamine transporter gene SLC6A3 carries a 5′ distal 5-kb super enhancer (5KSE) which upregulated the promoter by 5-fold. Interestingly, 5KSE is able to prevent 3′ downstream variable number tandem repeats (3'VNTRs) from silencing the promoter. This new enhancer consists of a 5′VNTR and three repetitive sub-elements that are conserved in primates. Two of 5KSE’s sub-elements, E-9.7 and E-8.7, upregulate the promoter, but only the later could continue doing so in the presence of 3'VNTRs. Finally, E-8.7 is activated by novel dopaminergic transcription factors including SRP54 and Nfe2l1. Together, these results reveal a multimodal regulatory mechanism in SLC6A3.





Similar content being viewed by others
References
Hughes JR, Roberts N, McGowan S, Hay D, Giannoulatou E, Lynch M, De Gobbi M, Taylor S et al (2014) Analysis of hundreds of cis-regulatory landscapes at high resolution in a single, high-throughput experiment. Nat Genet 46(2):205–212. https://doi.org/10.1038/ng.2871
Maston GA, Evans SK, Green MR (2006) Transcriptional regulatory elements in the human genome. Annu Rev Genomics Hum Genet 7:29–59. https://doi.org/10.1146/annurev.genom.7.080505.115623
Noonan JP, McCallion AS (2010) Genomics of long-range regulatory elements. Annu Rev Genomics Hum Genet 11:1–23. https://doi.org/10.1146/annurev-genom-082509-141651
Sudmant PH, Rausch T, Gardner EJ, Handsaker RE, Abyzov A, Huddleston J, Zhang Y, Ye K et al (2015) An integrated map of structural variation in 2,504 human genomes. Nature 526(7571):75–81. https://doi.org/10.1038/nature15394
Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S et al (2015) A global reference for human genetic variation. Nature 526(7571):68–74. https://doi.org/10.1038/nature15393
Jaber M, Jones S, Giros B, Caron MG (1997) The dopamine transporter: a crucial component regulating dopamine transmission. Mov Disord: Off J Mov Disord Soc 12(5):629–633. https://doi.org/10.1002/mds.870120502
Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K, Gether U (2011) SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev 63(3):585–640. https://doi.org/10.1124/pr.108.000869
Amara SG, Kuhar MJ (1993) Neurotransmitter transporters: recent progress. Annu Rev Neurosci 16:73–93. https://doi.org/10.1146/annurev.ne.16.030193.000445
Lin Z, Canales JJ, Bjorgvinsson T, Thomsen M, Qu H, Liu QR, Torres GE, Caine SB (2011) Monoamine transporters: vulnerable and vital doorkeepers. Prog Mol Biol Transl Sci 98:1–46. https://doi.org/10.1016/b978-0-12-385506-0.00001-6
Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, Uhl GR (1992) Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics 14(4):1104–1106
Guindalini C, Howard M, Haddley K, Laranjeira R, Collier D, Ammar N, Craig I, O’Gara C et al (2006) A dopamine transporter gene functional variant associated with cocaine abuse in a Brazilian sample. Proc Natl Acad Sci U S A 103(12):4552–4557. https://doi.org/10.1073/pnas.0504789103
Franke B, Vasquez AA, Johansson S, Hoogman M, Romanos J, Boreatti-Hummer A, Heine M, Jacob CP, Lesch KP, Casas M, Ribases M, Bosch R, Sanchez-Mora C, Gomez-Barros N, Fernandez-Castillo N, Bayes M, Halmoy A, Halleland H, Landaas ET, Fasmer OB, Knappskog PM, Heister AJ, Kiemeney LA, Kooij JJ, Boonstra AM, Kan CC, Asherson P, Faraone SV, Buitelaar JK, Haavik J, Cormand B, Ramos-Quiroga JA, Reif A (2010) Multicenter analysis of the SLC6A3/DAT1 VNTR haplotype in persistent ADHD suggests differential involvement of the gene in childhood and persistent ADHD. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 35 (3):656–664. doi:https://doi.org/10.1038/npp.2009.170
Zhao Y, Xiong N, Liu Y, Zhou Y, Li N, Qing H, Lin Z (2013) Human dopamine transporter gene: differential regulation of 18-kb haplotypes. Pharmacogenomics 14(12):1481–1494. https://doi.org/10.2217/pgs.13.141
Fuke S, Sasagawa N, Ishiura S (2005) Identification and characterization of the Hesr1/Hey1 as a candidate trans-acting factor on gene expression through the 3′ non-coding polymorphic region of the human dopamine transporter (DAT1) gene. J Biochem 137(2):205–216. https://doi.org/10.1093/jb/mvi020
Sacchetti P, Brownschidle LA, Granneman JG, Bannon MJ (1999) Characterization of the 5′-flanking region of the human dopamine transporter gene. Brain Res Mol Brain Res 74(1–2):167–174
Zhao Y, Zhou Y, Xiong N, Lin Z (2012) Identification of an intronic cis-acting element in the human dopamine transporter gene. Mol Biol Rep 39(5):5393–5399. https://doi.org/10.1007/s11033-011-1339-4
Kouzmenko AP, Pereira AM, Singh BS (1997) Intronic sequences are involved in neural targeting of human dopamine transporter gene expression. Biochem Biophys Res Commun 240(3):807–811. https://doi.org/10.1006/bbrc.1997.7754
Liu K, Yu J, Zhao J, Zhou Y, Xiong N, Xu J, Wang T, Bell RL, Qing H, Lin Z (2017) (AZI2)3’UTR is a new SLC6A3 downregulator associated with an epistatic protection against substance use disorders. Mol Neurobiol doi:https://doi.org/10.1007/s12035-017-0781-2, 55, 5611, 5622
Sacchetti P, Mitchell TR, Granneman JG, Bannon MJ (2001) Nurr1 enhances transcription of the human dopamine transporter gene through a novel mechanism. J Neurochem 76(5):1565–1572
Wang J, Bannon MJ (2005) Sp1 and Sp3 activate transcription of the human dopamine transporter gene. J Neurochem 93(2):474–482. https://doi.org/10.1111/j.1471-4159.2005.03051.x
Martinat C, Bacci JJ, Leete T, Kim J, Vanti WB, Newman AH, Cha JH, Gether U et al (2006) Cooperative transcription activation by Nurr1 and Pitx3 induces embryonic stem cell maturation to the midbrain dopamine neuron phenotype. Proc Natl Acad Sci U S A 103(8):2874–2879. https://doi.org/10.1073/pnas.0511153103
Stotz A, Linder P (1990) The ADE2 gene from Saccharomyces cerevisiae: sequence and new vectors. Gene 95(1):91–98
Fromont-Racine M, Rain JC, Legrain P (1997) Toward a functional analysis of the yeast genome through exhaustive two-hybrid screens. Nat Genet 16(3):277–282. https://doi.org/10.1038/ng0797-277
Kennedy JL, Xiong N, Yu J, Zai CC, Pouget JG, Li J, Liu K, Qing H et al (2016) Increased Nigral SLC6A3 activity in schizophrenia patients: findings from the Toronto-McLean cohorts. Schizophr Bull 42(3):772–781. https://doi.org/10.1093/schbul/sbv191
Hannan AJ (2018) Tandem repeats mediating genetic plasticity in health and disease. Nat Rev Genet 19:286–298. https://doi.org/10.1038/nrg.2017.115
Zhou Y, Michelhaugh SK, Schmidt CJ, Liu JS, Bannon MJ, Lin Z (2014) Ventral midbrain correlation between genetic variation and expression of the dopamine transporter gene in cocaine-abusing versus non-abusing subjects. Addict Biol 19(1):122–131. https://doi.org/10.1111/j.1369-1600.2011.00391.x
Li S, Kim KY, Kim JH, Kim JH, Park MS, Bahk JY, Kim MO (2004) Chronic nicotine and smoking treatment increases dopamine transporter mRNA expression in the rat midbrain. Neurosci Lett 363(1):29–32. https://doi.org/10.1016/j.neulet.2004.03.053
Hadjiconstantinou M, Duchemin AM, Zhang H, Neff NH (2011) Enhanced dopamine transporter function in striatum during nicotine withdrawal. Synapse (New York, NY) 65(2):91–98. https://doi.org/10.1002/syn.20820
Filipenko ML, Alekseyenko OV, Beilina AG, Kamynina TP, Kudryavtseva NN (2001) Increase of tyrosine hydroxylase and dopamine transporter mRNA levels in ventral tegmental area of male mice under influence of repeated aggression experience. Brain Res Mol Brain Res 96(1–2):77–81
Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y (2016) Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol 41(1):335–356. https://doi.org/10.1038/npp.2015.142
Ong ZY, Muhlhausler BS (2011) Maternal “junk-food” feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. FASEB J: Off Publ Fed Am Soc Exp Biol 25(7):2167–2179. https://doi.org/10.1096/fj.10-178392
Kandel ER, Kandel DB (2014) Shattuck lecture. A molecular basis for nicotine as a gateway drug. N Engl J Med 371(10):932–943. https://doi.org/10.1056/NEJMsa1405092
Amendt BA, Sutherland LB, Russo AF (1999) Transcriptional antagonism between Hmx1 and Nkx2.5 for a shared DNA-binding site. The J Biol Chem 274 (17):11635–11642
Bougarne N, Paumelle R, Caron S, Hennuyer N, Mansouri R, Gervois P, Staels B, Haegeman G, De Bosscher K (2009) PPARalpha blocks glucocorticoid receptor alpha-mediated transactivation but cooperates with the activated glucocorticoid receptor alpha for transrepression on NF-kappaB. Proc Natl Acad Sci U S A 106 (18):7397–7402. doi:https://doi.org/10.1073/pnas.0806742106
Hoppe KL, Francone OL (1998) Binding and functional effects of transcription factors Sp1 and Sp3 on the proximal human lecithin:cholesterol acyltransferase promoter. J Lipid Res 39(5):969–977
Ilsley MD, Gillinder KR, Magor GW, Huang S, Bailey TL, Crossley M, Perkins AC (2017) Krüppel-like factors compete for promoters and enhancers to fine-tune transcription. Nucleic Acids Res 45(11):6572–6588. https://doi.org/10.1093/nar/gkx441
Joseph SR, Palfy M, Hilbert L, Kumar M, Karschau J, Zaburdaev V, Shevchenko A, Vastenhouw NL (2017) Competition between histone and transcription factor binding regulates the onset of transcription in zebrafish embryos. eLife 6. https://doi.org/10.7554/eLife.23326
Pierce SL, England SK (2010) SK3 channel expression during pregnancy is regulated through estrogen and Sp factor-mediated transcriptional control of the KCNN3 gene. Am J Phys Endocrinol Metab 299(4):E640–E646. https://doi.org/10.1152/ajpendo.00063.2010
Culverhouse R, Suarez BK, Lin J, Reich T (2002) A perspective on epistasis: limits of models displaying no main effect. Am J Hum Genet 70(2):461–471. https://doi.org/10.1086/338759
Hemani G, Knott S, Haley C (2013) An evolutionary perspective on epistasis and the missing heritability. PLoS Genet 9(2):e1003295. https://doi.org/10.1371/journal.pgen.1003295
Zuk O, Hechter E, Sunyaev SR, Lander ES (2012) The mystery of missing heritability: genetic interactions create phantom heritability. Proc Natl Acad Sci U S A 109(4):1193–1198. https://doi.org/10.1073/pnas.1119675109
Fujita M, Shimada S, Nishimura T, Uhl GR, Tohyama M (1993) Ontogeny of dopamine transporter mRNA expression in the rat brain. Brain Res Mol Brain Res 19(3):222–226
Nord AS, Blow MJ, Attanasio C, Akiyama JA, Holt A, Hosseini R, Phouanenavong S, Plajzer-Frick I et al (2013) Rapid and pervasive changes in genome-wide enhancer usage during mammalian development. Cell 155(7):1521–1531. https://doi.org/10.1016/j.cell.2013.11.033
Osterwalder M, Barozzi I, Tissieres V, Fukuda-Yuzawa Y, Mannion BJ, Afzal SY, Lee EA, Zhu Y et al (2018) Enhancer redundancy provides phenotypic robustness in mammalian development. Nature 554(7691):239–243. https://doi.org/10.1038/nature25461
Villaescusa JC, Li B, Toledo EM, Rivetti di Val Cervo P, Yang S, Stott SR, Kaiser K, Islam S et al (2016) A PBX1 transcriptional network controls dopaminergic neuron development and is impaired in Parkinson’s disease. EMBO J 35(18):1963–1978. https://doi.org/10.15252/embj.201593725
Janda CY, Li J, Oubridge C, Hernandez H, Robinson CV, Nagai K (2010) Recognition of a signal peptide by the signal recognition particle. Nature 465(7297):507–510. https://doi.org/10.1038/nature08870
Morrish F, Giedt C, Hockenbery D (2003) c-MYC apoptotic function is mediated by NRF-1 target genes. Genes Dev 17(2):240–255. https://doi.org/10.1101/gad.1032503
Morikawa M, Koinuma D, Tsutsumi S, Vasilaki E, Kanki Y, Heldin CH, Aburatani H, Miyazono K (2011) ChIP-seq reveals cell type-specific binding patterns of BMP-specific Smads and a novel binding motif. Nucleic Acids Res 39(20):8712–8727. https://doi.org/10.1093/nar/gkr572
Gertz J, Reddy TE, Varley KE, Garabedian MJ, Myers RM (2012) Genistein and bisphenol A exposure cause estrogen receptor 1 to bind thousands of sites in a cell type-specific manner. Genome Res 22(11):2153–2162. https://doi.org/10.1101/gr.135681.111
Funding
This work is supported by NIH grants DA021409 and DA031573 to ZL; YZ, JZ, XC, and NX were supported by Chinese Government Visiting Scholarships.
Author information
Authors and Affiliations
Contributions
ZL, TW, and HQ designed study; YZ, JY, JZ, XC, NX, and ZL participated in experiments and data analysis; and ZL wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhao, Y., Yu, J., Zhao, J. et al. Intragenic Transcriptional cis-Antagonism Across SLC6A3. Mol Neurobiol 56, 4051–4060 (2019). https://doi.org/10.1007/s12035-018-1357-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1357-5