Abstract
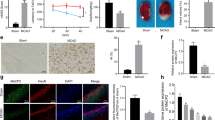
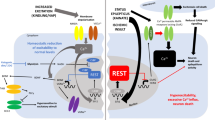
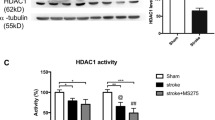
Cerebral ischemia is known to activate the repressor element-1 (RE1)-silencing transcription factor (REST) which silences neural genes via epigenetic remodeling and promotes neurodegeneration. We presently determined if REST inhibition derepresses target genes involved in synaptic plasticity and promotes functional outcome after experimental stroke. Following transient focal ischemia induced by middle cerebral artery occlusion (MCAO) in adult rats, REST expression was upregulated significantly from 12 h to 1 day of reperfusion compared to sham control. At 1 day of reperfusion, REST protein levels were increased and observed in the nuclei of neurons in the peri-infarct cortex. REST knockdown by intracerebral REST siRNA injection significantly reduced the post-ischemic expression of REST and increased the expression of several REST target genes, compared to control siRNA group. REST inhibition also decreased post-ischemic markers of apoptosis, reduced cortical infarct volume, and improved post-ischemic functional recovery on days 5 and 7 of reperfusion compared to the control siRNA group. REST knockdown resulted in a global increase in synaptic plasticity gene expression at 1 day of reperfusion compared to the control siRNA group and significantly increased several synaptic plasticity genes containing RE-1 sequences in their regulatory regions. These results demonstrate that direct inhibition of the epigenetic remodeler REST prevents secondary brain damage in the cortex and improves functional outcome potentially via de-repression of plasticity-related genes after stroke.





Similar content being viewed by others
References
Hu Z, Zhong B, Tan J, Chen C, Lei Q, Zeng L (2016) The emerging role of epigenetics in cerebral ischemia. Mol Neurobiol 54:1887–1905. https://doi.org/10.1007/s12035-016-9788-3
Calderone A, Jover T, Noh KM, Tanaka H, Yokota H, Lin Y, Grooms SY, Regis R et al (2003) Ischemic insults derepress the gene silencer REST in neurons destined to die. J Neurosci 23(6):2112–2121
Noh KM, Hwang JY, Follenzi A, Athanasiadou R, Miyawaki T, Greally JM, Bennett MV, Zukin RS (2012) Repressor element-1 silencing transcription factor (REST)-dependent epigenetic remodeling is critical to ischemia-induced neuronal death. Proc Natl Acad Sci U S A 109(16):E962–E971. https://doi.org/10.1073/pnas.1121568109
Mehta SL, Kim T, Vemuganti R (2015) Long noncoding RNA FosDT promotes ischemic brain injury by interacting with REST-associated chromatin-modifying proteins. J Neurosci 35(50):16443–16449. https://doi.org/10.1523/jneurosci.2943-15.2015
Liu Z, Liu M, Niu G, Cheng Y, Fei J (2009) Genome-wide identification of target genes repressed by the zinc finger transcription factor REST/NRSF in the HEK 293 cell line. Acta Biochim Biophys Sin Shanghai 41(12):1008–1017
Schoenherr CJ, Paquette AJ, Anderson DJ (1996) Identification of potential target genes for the neuron-restrictive silencer factor. Proc Natl Acad Sci U S A 93(18):9881–9886
Huang Y, Myers SJ, Dingledine R (1999) Transcriptional repression by REST: recruitment of Sin3A and histone deacetylase to neuronal genes. Nat Neurosci 2(10):867–872. https://doi.org/10.1038/13165
Qureshi IA, Mehler MF (2009) Regulation of non-coding RNA networks in the nervous system—what’s the REST of the story? Neurosci Lett 466(2):73–80. https://doi.org/10.1016/j.neulet.2009.07.093
Wu J, Xie X (2006) Comparative sequence analysis reveals an intricate network among REST, CREB and miRNA in mediating neuronal gene expression. Genome Biol 7(9):R85. https://doi.org/10.1186/gb-2006-7-9-r85
Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G (2005) REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell 121(4):645–657. https://doi.org/10.1016/j.cell.2005.03.013
Abrajano JJ, Qureshi IA, Gokhan S, Molero AE, Zheng D, Bergman A, Mehler MF (2010) Corepressor for element-1-silencing transcription factor preferentially mediates gene networks underlying neural stem cell fate decisions. Proc Natl Acad Sci U S A 107(38):16685–16690. https://doi.org/10.1073/pnas.0906917107
Zuccato C, Tartari M, Crotti A, Goffredo D, Valenza M, Conti L, Cataudella T, Leavitt BR et al (2003) Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet 35(1):76–83. https://doi.org/10.1038/ng1219
Paquette AJ, Perez SE, Anderson DJ (2000) Constitutive expression of the neuron-restrictive silencer factor (NRSF)/REST in differentiating neurons disrupts neuronal gene expression and causes axon pathfinding errors in vivo. Proc Natl Acad Sci U S A 97(22):12318–12323. https://doi.org/10.1073/pnas.97.22.12318
Baldelli P, Meldolesi J (2015) The transcription repressor REST in adult neurons: physiology, pathology, and diseases (1,2,3). eNeuro 2(4). doi:https://doi.org/10.1523/eneuro.0010-15.2015
Lu T, Aron L, Zullo J, Pan Y, Kim H, Chen Y, Yang TH, Kim HM et al (2014) REST and stress resistance in ageing and Alzheimer’s disease. Nature 507(7493):448–454. https://doi.org/10.1038/nature13163
Zuccato C, Belyaev N, Conforti P, Ooi L, Tartari M, Papadimou E, MacDonald M, Fossale E et al (2007) Widespread disruption of repressor element-1 silencing transcription factor/neuron-restrictive silencer factor occupancy at its target genes in Huntington’s disease. J Neurosci 27(26):6972–6983. https://doi.org/10.1523/jneurosci.4278-06.2007
Ooi L, Wood IC (2007) Chromatin crosstalk in development and disease: lessons from REST. Nat Rev Genet 8(7):544–554. https://doi.org/10.1038/nrg2100
McClelland S, Flynn C, Dube C, Richichi C, Zha Q, Ghestem A, Esclapez M, Bernard C et al (2011) Neuron-restrictive silencer factor-mediated hyperpolarization-activated cyclic nucleotide gated channelopathy in experimental temporal lobe epilepsy. Ann Neurol 70(3):454–464. https://doi.org/10.1002/ana.22479
Lin TP, Chang YT, Lee SY, Campbell M, Wang TC, Shen SH, Chung HJ, Chang YH et al (2016) REST reduction is essential for hypoxia-induced neuroendocrine differentiation of prostate cancer cells by activating autophagy signaling. Oncotarget 7(18):26137–26151. https://doi.org/10.18632/oncotarget.8433
Cavadas MA, Mesnieres M, Crifo B, Manresa MC, Selfridge AC, Scholz CC, Cummins EP, Cheong A et al (2015) REST mediates resolution of HIF-dependent gene expression in prolonged hypoxia. Sci Rep 5:17851. https://doi.org/10.1038/srep17851
Cavadas MA, Mesnieres M, Crifo B, Manresa MC, Selfridge AC, Keogh CE, Fabian Z, Scholz CC et al (2016) REST is a hypoxia-responsive transcriptional repressor. Sci Rep 6:31355. https://doi.org/10.1038/srep31355
Pandi G, Nakka VP, Dharap A, Roopra A, Vemuganti R (2013) MicroRNA miR-29c down-regulation leading to de-repression of its target DNA methyltransferase 3a promotes ischemic brain damage. PLoS One 8(3):e58039. https://doi.org/10.1371/journal.pone.0058039
Formisano L, Guida N, Valsecchi V, Cantile M, Cuomo O, Vinciguerra A, Laudati G, Pignataro G et al (2015) Sp3/REST/HDAC1/HDAC2 complex represses and Sp1/HIF-1/p300 complex activates ncx1 gene transcription, in brain ischemia and in ischemic brain preconditioning, by epigenetic mechanism. J Neurosci 35(19):7332–7348. https://doi.org/10.1523/jneurosci.2174-14.2015
Formisano L, Guida N, Valsecchi V, Pignataro G, Vinciguerra A, Pannaccione A, Secondo A, Boscia F et al (2013) NCX1 is a new rest target gene: role in cerebral ischemia. Neurobiol Dis 50:76–85. https://doi.org/10.1016/j.nbd.2012.10.010
Nakka VP, Lang BT, Lenschow DJ, Zhang DE, Dempsey RJ, Vemuganti R (2011) Increased cerebral protein ISGylation after focal ischemia is neuroprotective. J Cereb Blood Flow Metab 31(12):2375–2384. https://doi.org/10.1038/jcbfm.2011.103
Mehta SL, Pandi G, Vemuganti R (2017) Circular RNA expression profiles alter significantly in mouse brain after transient focal ischemia. Stroke 48(9):2541–2548. https://doi.org/10.1161/strokeaha.117.017469
Shyu WC, Lin SZ, Chiang MF, Chen DC, Su CY, Wang HJ, Liu RS, Tsai CH et al (2008) Secretoneurin promotes neuroprotection and neuronal plasticity via the Jak2/Stat3 pathway in murine models of stroke. J Clin Invest 118(1):133–148. https://doi.org/10.1172/jci32723
Murphy TH, Corbett D (2009) Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci 10(12):861–872. https://doi.org/10.1038/nrn2735
Pekna M, Pekny M, Nilsson M (2012) Modulation of neural plasticity as a basis for stroke rehabilitation. Stroke 43(10):2819–2828. https://doi.org/10.1161/strokeaha.112.654228
Johnson DS, Mortazavi A, Myers RM, Wold B (2007) Genome-wide mapping of in vivo protein-DNA interactions. Science 316(5830):1497–1502. https://doi.org/10.1126/science.1141319
Mortazavi A, Leeper Thompson EC, Garcia ST, Myers RM, Wold B (2006) Comparative genomics modeling of the NRSF/REST repressor network: from single conserved sites to genome-wide repertoire. Genome Res 16(10):1208–1221. https://doi.org/10.1101/gr.4997306
Bruce AW, Donaldson IJ, Wood IC, Yerbury SA, Sadowski MI, Chapman M, Gottgens B, Buckley NJ (2004) Genome-wide analysis of repressor element 1 silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) target genes. Proc Natl Acad Sci U S A 101(28):10458–10463. https://doi.org/10.1073/pnas.0401827101
Johnson R, Teh CH, Kunarso G, Wong KY, Srinivasan G, Cooper ML, Volta M, Chan SS et al (2008) REST regulates distinct transcriptional networks in embryonic and neural stem cells. PLoS Biol 6(10):e256. https://doi.org/10.1371/journal.pbio.0060256
Yu M, Cai L, Liang M, Huang Y, Gao H, Lu S, Fei J, Huang F (2009) Alteration of NRSF expression exacerbating 1-methyl-4-phenyl-pyridinium ion-induced cell death of SH-SY5Y cells. Neurosci Res 65(3):236–244. https://doi.org/10.1016/j.neures.2009.07.006
Yu M, Suo H, Liu M, Cai L, Liu J, Huang Y, Xu J, Wang Y et al (2013) NRSF/REST neuronal deficient mice are more vulnerable to the neurotoxin MPTP. Neurobiol Aging 34(3):916–927. https://doi.org/10.1016/j.neurobiolaging.2012.06.002
Posod A, Wechselberger K, Stanika RI, Obermair GJ, Wegleiter K, Huber E, Urbanek M, Kiechl-Kohlendorfer U et al (2017) Administration of secretoneurin is protective in hypoxic-ischemic neonatal brain injury predominantly in the hypoxic-only hemisphere. Neuroscience 352:88–96. https://doi.org/10.1016/j.neuroscience.2017.03.055
Oguro K, Oguro N, Kojima T, Grooms SY, Calderone A, Zheng X, Bennett MV, Zukin RS (1999) Knockdown of AMPA receptor GluR2 expression causes delayed neurodegeneration and increases damage by sublethal ischemia in hippocampal CA1 and CA3 neurons. J Neurosci 19(21):9218–9227
Naruse S, Aoki Y, Takei R, Horikawa Y, Ueda S (1991) Effects of atrial natriuretic peptide on ischemic brain edema in rats evaluated by proton magnetic resonance method. Stroke 22(1):61–65
Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M (1999) NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med 5(5):554–559. https://doi.org/10.1038/8432
Lenz M, Vlachos A, Maggio N (2015) Ischemic long-term-potentiation (iLTP): perspectives to set the threshold of neural plasticity toward therapy. Neural Regen Res 10(10):1537–1539. https://doi.org/10.4103/1673-5374.165215
Minatohara K, Akiyoshi M, Okuno H (2015) Role of immediate-early genes in synaptic plasticity and neuronal ensembles underlying the memory trace. Front Mol Neurosci 8:78. doi:https://doi.org/10.3389/fnmol.2015.00078
Lanahan A, Worley P (1998) Immediate-early genes and synaptic function. Neurobiol Learn Mem 70(1–2):37–43. https://doi.org/10.1006/nlme.1998.3836
Schabitz WR, Berger C, Kollmar R, Seitz M, Tanay E, Kiessling M, Schwab S, Sommer C (2004) Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke 35(4):992–997. https://doi.org/10.1161/01.str.0000119754.85848.0d
Schabitz WR, Sommer C, Zoder W, Kiessling M, Schwaninger M, Schwab S (2000) Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke 31(9):2212–2217
Berretta A, Tzeng YC, Clarkson AN (2014) Post-stroke recovery: the role of activity-dependent release of brain-derived neurotrophic factor. Expert Rev Neurother 14(11):1335–1344. https://doi.org/10.1586/14737175.2014.969242
Lu B, Nagappan G, Lu Y (2014) BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol 220:223–250. https://doi.org/10.1007/978-3-642-45106-5_9
Glorioso C, Sabatini M, Unger T, Hashimoto T, Monteggia LM, Lewis DA, Mirnics K (2006) Specificity and timing of neocortical transcriptome changes in response to BDNF gene ablation during embryogenesis or adulthood. Mol Psychiatry 11(7):633–648. https://doi.org/10.1038/sj.mp.4001835
Jourdi H, Kabbaj M (2013) Acute BDNF treatment upregulates GluR1-SAP97 and GluR2-GRIP1 interactions: implications for sustained AMPA receptor expression. PLoS One 8(2):e57124. https://doi.org/10.1371/journal.pone.0057124
Sato M, Suzuki K, Nakanishi S (2001) NMDA receptor stimulation and brain-derived neurotrophic factor upregulate homer 1a mRNA via the mitogen-activated protein kinase cascade in cultured cerebellar granule cells. J Neurosci 21(11):3797–3805
Wu CL, Yin JH, Hwang CS, Chen SD, Yang DY, Yang DI (2012) c-Jun-dependent sulfiredoxin induction mediates BDNF protection against mitochondrial inhibition in rat cortical neurons. Neurobiol Dis 46(2):450–462. https://doi.org/10.1016/j.nbd.2012.02.010
Kuzniewska B, Rejmak E, Malik AR, Jaworski J, Kaczmarek L, Kalita K (2013) Brain-derived neurotrophic factor induces matrix metalloproteinase 9 expression in neurons via the serum response factor/c-Fos pathway. Mol Cell Biol 33(11):2149–2162. https://doi.org/10.1128/mcb.00008-13
Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H (2004) Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci 24(44):9760–9769. https://doi.org/10.1523/jneurosci.1427-04.2004
Funding
This study was funded by the National Institute of Health grant no. R21NS095192, RO1 NS099531, and RO1 NS101960.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
All surgical procedures were approved by the Research Animal Resources and Care Committee of the University of Wisconsin-Madison, and the rats were cared for in accordance with the Guide for the Care and Use of Laboratory Animals (U.S. Department of Health and Human Services Publication 86-23, revised).
Rights and permissions
About this article
Cite this article
Morris-Blanco, K.C., Kim, T., Bertogliat, M.J. et al. Inhibition of the Epigenetic Regulator REST Ameliorates Ischemic Brain Injury. Mol Neurobiol 56, 2542–2550 (2019). https://doi.org/10.1007/s12035-018-1254-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1254-y