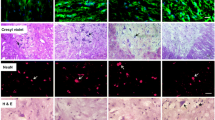
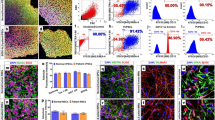
Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by motor neuron (MN) loss. Recent evidences highlight astrocytes as important players in MN death, but the mechanism-based neurotoxicity is still unknown. It is also unclear whether activation of astrocytes in ALS occurs differently in the cerebral cortex and spinal cord. We investigated glial and neuronal alterations in the cortex of SOD1G93A (mSOD1) mice in pre-symptomatic and symptomatic stages. We also characterized astrocytes isolated from the cortex of 7-day-old mSOD1 mice for their aberrancy and MN-induced degenerative effects. In the early stage, we identified a reduction of cell proliferation, NF-kB expression, and of vimentin and micro(miR)-146a expression, suggesting a restrained cortical inflammatory status. However, increased NF-kB expression, cell proliferation, and gene expression of HMGB1, connexin 43 and S100B were distinctive of the symptomatic stage, together with MN loss, downregulated unfold protein response, and decreased expression of synaptic proteins, together with that of miR-125b, miR-21, miR-146a, GFAP, and glutamate transporters. Astrocytes cultured for 13 days in vitro showed comparable NF-kB expression and cell proliferation increase, as well as similar microRNA and gene/protein expression profiles (decreased miR-21, miR-146a, GLT-1 and GFAP, and upregulated HMGB1, S100B and connexin-43), thus sustaining astrocytes as the major contributors of cortical homeostasis deregulation in the symptomatic stage. These reactive astrocytes reduced neurite length and synaptophysin expression in NSC-34/hSOD1WT MN-like cells, and induced mitochondria dysfunction, PSD-95 downregulation, metalloproteinase-9 activation, and late apoptosis in NSC-34/hSOD1G93A cells. Data indicate that astrocytes in mSOD1 mice model acquire early phenotypic aberrancies and highlight downregulated miR-146a as a biomarker and drug target in ALS.








Similar content being viewed by others
Abbreviations
- ALS:
-
amyotrophic lateral sclerosis
- ATF4:
-
activating transcription factor 4
- ChAT:
-
choline acetyltransferase
- CNS:
-
central nervous system
- Cx43:
-
connexin-43
- DIV:
-
days in vitro
- eIF2α:
-
eukaryotic initiation factor 2 alpha
- ER:
-
endoplasmic reticulum
- fALS:
-
familial ALS
- GFAP:
-
glial fibrillary acidic protein
- GLAST:
-
glutamate aspartate transporter
- GLT-1:
-
glutamate transporter 1
- GluT:
-
glutamate transporters
- HMGB1:
-
high mobility group box 1
- IRAK1:
-
interleukin-1 receptor-associated kinase 1
- LC3B:
-
microtubule-associated protein light chain 3
- MBP:
-
myelin basic protein
- miR:
-
microRNA
- MMP:
-
matrix metalloproteinase
- MN:
-
motor neuron
- mSOD1:
-
mutant SOD1/SOD1G93A
- NeuN:
-
neuronal nuclei antigen
- NF-kB:
-
nuclear factor kappa B
- NG2:
-
neural/glial antigen 2
- ON:
-
overnight
- PBS:
-
phosphate buffer saline
- PFA:
-
paraformaldehyde
- PN:
-
postnatal
- PSD-95:
-
post-synaptic density protein 95
- qPCR:
-
quantitative real time-transcription polymerase chain reaction
- RT:
-
room temperature
- S100B:
-
S100 calcium binding protein B
- sALS:
-
sporadic ALS
- SC:
-
spinal cord
- SOD1:
-
superoxide dismutase 1
- SYP:
-
synaptophysin
- TRAF6:
-
tumor necrosis factor receptor-associated factor 6
- WT:
-
wild-type
References
Bento-Abreu A, Van Damme P, Van Den Bosch L, Robberecht W (2010) The neurobiology of amyotrophic lateral sclerosis. Eur J Neurosci 31(12):2247–2265. https://doi.org/10.1111/j.1460-9568.2010.07260.x
Rosen DR (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 364(6435):362. https://doi.org/10.1038/364362c0
Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A et al (1994) Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264(5166):1772–1775
Pfohl SR, Halicek MT, Mitchell CS (2015) Characterization of the contribution of genetic background and gender to disease progression in the SOD1 G93A mouse model of amyotrophic lateral sclerosis: a meta-analysis. J Neuromuscul Dis 2(2):137–150. https://doi.org/10.3233/JND-140068
McCombe PA, Henderson RD (2011) The role of immune and inflammatory mechanisms in ALS. Curr Mol Med 11(3):246–254
Saxena S, Caroni P (2011) Selective neuronal vulnerability in neurodegenerative diseases: from stressor thresholds to degeneration. Neuron 71(1):35–48. https://doi.org/10.1016/j.neuron.2011.06.031
Gruzman A, Wood WL, Alpert E, Prasad MD, Miller RG, Rothstein JD, Bowser R, Hamilton R et al (2007) Common molecular signature in SOD1 for both sporadic and familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 104(30):12524–12529. https://doi.org/10.1073/pnas.0705044104
Wang Q, Johnson JL, Agar NY, Agar JN (2008) Protein aggregation and protein instability govern familial amyotrophic lateral sclerosis patient survival. PLoS Biol 6(7):e170. https://doi.org/10.1371/journal.pbio.0060170
Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, McMahon AP, Doucette W et al (2003) Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science 302(5642):113–117. https://doi.org/10.1126/science.1086071
Forsberg K, Andersen PM, Marklund SL, Brannstrom T (2011) Glial nuclear aggregates of superoxide dismutase-1 are regularly present in patients with amyotrophic lateral sclerosis. Acta Neuropathol 121(5):623–634. https://doi.org/10.1007/s00401-011-0805-3
Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H et al (2008) Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci 11(3):251–253. https://doi.org/10.1038/nn2047
Papadeas ST, Kraig SE, O'Banion C, Lepore AC, Maragakis NJ (2011) Astrocytes carrying the superoxide dismutase 1 (SOD1G93A) mutation induce wild-type motor neuron degeneration in vivo. Proc Natl Acad Sci U S A 108(43):17803–17808. https://doi.org/10.1073/pnas.1103141108
Diaz-Amarilla P, Olivera-Bravo S, Trias E, Cragnolini A, Martinez-Palma L, Cassina P, Beckman J, Barbeito L (2011) Phenotypically aberrant astrocytes that promote motoneuron damage in a model of inherited amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 108(44):18126–18131. https://doi.org/10.1073/pnas.1110689108
Meyer K, Ferraiuolo L, Miranda CJ, Likhite S, McElroy S, Renusch S, Ditsworth D, Lagier-Tourenne C et al (2014) Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proc Natl Acad Sci U S A 111(2):829–832. https://doi.org/10.1073/pnas.1314085111
Kamo H, Haebara H, Akiguchi I, Kameyama M, Kimura H, McGeer PL (1987) A distinctive distribution of reactive astroglia in the precentral cortex in amyotrophic lateral sclerosis. Acta Neuropathol 74(1):33–38
Nagy D, Kato T, Kushner PD (1994) Reactive astrocytes are widespread in the cortical gray matter of amyotrophic lateral sclerosis. J Neurosci Res 38(3):336–347. https://doi.org/10.1002/jnr.490380312
Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S (2007) Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci 10(5):615–622. https://doi.org/10.1038/nn1876
Almad AA, Doreswamy A, Gross SK, Richard JP, Huo Y, Haughey N, Maragakis NJ (2016) Connexin 43 in astrocytes contributes to motor neuron toxicity in amyotrophic lateral sclerosis. Glia 64(7):1154–1169. https://doi.org/10.1002/glia.22989
Cunha C, Santos C, Gomes C, Fernandes A, Correia AM, Sebastiao AM, Vaz AR, Brites D (2017) Downregulated glia interplay and increased miRNA-155 as promising markers to track ALS at an early stage. Mol Neurobiol. https://doi.org/10.1007/s12035-12017-10631-12032
Parisi C, Arisi I, D'Ambrosi N, Storti AE, Brandi R, D'Onofrio M, Volonte C (2013) Dysregulated microRNAs in amyotrophic lateral sclerosis microglia modulate genes linked to neuroinflammation. Cell Death Dis 4:e959. https://doi.org/10.1038/cddis.2013.491
Koval ED, Shaner C, Zhang P, du Maine X, Fischer K, Tay J, Chau BN, Wu GF et al (2013) Method for widespread microRNA-155 inhibition prolongs survival in ALS-model mice. Hum Mol Genet 22(20):4127–4135. https://doi.org/10.1093/hmg/ddt261
Butovsky O, Jedrychowski MP, Cialic R, Krasemann S, Murugaiyan G, Fanek Z, Greco DJ, Wu PM et al (2015) Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann Neurol 77(1):75–99. https://doi.org/10.1002/ana.24304
Ben Haim L, Carrillo-de Sauvage MA, Ceyzeriat K, Escartin C (2015) Elusive roles for reactive astrocytes in neurodegenerative diseases. Front Cell Neurosci 9:278. https://doi.org/10.3389/fncel.2015.00278
Sison SL, Patitucci TN, Seminary ER, Villalon E, Lorson CL, Ebert AD (2017) Astrocyte-produced miR-146a as a mediator of motor neuron loss in spinal muscular atrophy. Hum Mol Genet 26(17):3409–3420. https://doi.org/10.1093/hmg/ddx230
Trias E, Ibarburu S, Barreto-Nunez R, Barbeito L (2017) Significance of aberrant glial cell phenotypes in pathophysiology of amyotrophic lateral sclerosis. Neurosci Lett 636:27–31. https://doi.org/10.1016/j.neulet.2016.07.052
Rocha MC, Pousinha PA, Correia AM, Sebastiao AM, Ribeiro JA (2013) Early changes of neuromuscular transmission in the SOD1(G93A) mice model of ALS start long before motor symptoms onset. PLoS One 8(9):e73846. https://doi.org/10.1371/journal.pone.0073846
Nascimento F, Pousinha PA, Correia AM, Gomes R, Sebastiao AM, Ribeiro JA (2014) Adenosine A2A receptors activation facilitates neuromuscular transmission in the pre-symptomatic phase of the SOD1(G93A) ALS mice, but not in the symptomatic phase. PLoS One 9(8):e104081. https://doi.org/10.1371/journal.pone.0104081
Fernandes A, Barateiro A, Falcão AS, Silva SL, Vaz AR, Brito MA, Silva RF, Brites D (2011) Astrocyte reactivity to unconjugated bilirubin requires TNF-alpha and IL-1beta receptor signaling pathways. Glia 59(1):14–25. https://doi.org/10.1002/glia.21072
Vaz AR, Cunha C, Gomes C, Schmucki N, Barbosa M, Brites D (2015) Glycoursodeoxycholic acid reduces matrix metalloproteinase-9 and caspase-9 activation in a cellular model of superoxide dismutase-1 neurodegeneration. Mol Neurobiol 51(3):864–877. https://doi.org/10.1007/s12035-014-8731-8
Phatnani HP, Guarnieri P, Friedman BA, Carrasco MA, Muratet M, O'Keeffe S, Nwakeze C, Pauli-Behn F et al (2013) Intricate interplay between astrocytes and motor neurons in ALS. Proc Natl Acad Sci U S A 110(8):E756–E765. https://doi.org/10.1073/pnas.1222361110
Wang L, Popko B, Roos RP (2014) An enhanced integrated stress response ameliorates mutant SOD1-induced ALS. Hum Mol Genet 23(10):2629–2638. https://doi.org/10.1093/hmg/ddt658
Kim HJ, Raphael AR, LaDow ES, McGurk L, Weber RA, Trojanowski JQ, Lee VM, Finkbeiner S et al (2014) Therapeutic modulation of eIF2alpha phosphorylation rescues TDP-43 toxicity in amyotrophic lateral sclerosis disease models. Nat Genet 46(2):152–160. https://doi.org/10.1038/ng.2853
B'Chir W, Maurin AC, Carraro V, Averous J, Jousse C, Muranishi Y, Parry L, Stepien G et al (2013) The eIF2alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res 41(16):7683–7699. https://doi.org/10.1093/nar/gkt563
Sakowski SA, Lunn JS, Busta AS, Oh SS, Zamora-Berridi G, Palmer M, Rosenberg AA, Philip SG et al (2012) Neuromuscular effects of G93A-SOD1 expression in zebrafish. Mol Neurodegener 7:44. https://doi.org/10.1186/1750-1326-7-44
Pasini S, Corona C, Liu J, Greene LA, Shelanski ML (2015) Specific downregulation of hippocampal ATF4 reveals a necessary role in synaptic plasticity and memory. Cell Rep 11(2):183–191. https://doi.org/10.1016/j.celrep.2015.03.025
Matus S, Lopez E, Valenzuela V, Nassif M, Hetz C (2013) Functional contribution of the transcription factor ATF4 to the pathogenesis of amyotrophic lateral sclerosis. PLoS One 8(7):e66672. https://doi.org/10.1371/journal.pone.0066672
Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S (1999) Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron 23(4):659–674
Savioz A, Leuba G, Vallet PG (2014) A framework to understand the variations of PSD-95 expression in brain aging and in Alzheimer’s disease. Ageing Res Rev 18:86–94. https://doi.org/10.1016/j.arr.2014.09.004
Hou Q, Ruan H, Gilbert J, Wang G, Ma Q, Yao WD, Man HY (2015) MicroRNA miR124 is required for the expression of homeostatic synaptic plasticity. Nat Commun 6:10045. https://doi.org/10.1038/ncomms10045
Le MT, Xie H, Zhou B, Chia PH, Rizk P, Um M, Udolph G, Yang H et al (2009) MicroRNA-125b promotes neuronal differentiation in human cells by repressing multiple targets. Mol Cell Biol 29(19):5290–5305. https://doi.org/10.1128/MCB.01694-08
Frakes AE, Ferraiuolo L, Haidet-Phillips AM, Schmelzer L, Braun L, Miranda CJ, Ladner KJ, Bevan AK et al (2014) Microglia induce motor neuron death via the classical NF-kappaB pathway in amyotrophic lateral sclerosis. Neuron 81(5):1009–1023. https://doi.org/10.1016/j.neuron.2014.01.013
Kaltschmidt B, Kaltschmidt C (2009) NF-kappa B in the nervous system. Cold Spring Harb Perspect Biol 1(3). https://doi.org/10.1101/cshperspect.a001271
Shih RH, Wang CY, Yang CM (2015) NF-kappaB signaling pathways in neurological inflammation: a mini review. Front Mol Neurosci 8. doi:https://doi.org/10.3389/Fnmol.2015.00077
Luan ZG, Zhang H, Yang PT, Ma XC, Zhang C, Guo RX (2010) HMGB1 activates nuclear factor-kappaB signaling by RAGE and increases the production of TNF-alpha in human umbilical vein endothelial cells. Immunobiology 215(12):956–962. https://doi.org/10.1016/j.imbio.2009.11.001
Lo Coco D, Veglianese P, Allievi E, Bendotti C (2007) Distribution and cellular localization of high mobility group box protein 1 (HMGB1) in the spinal cord of a transgenic mouse model of ALS. Neurosci Lett 412(1):73–77. https://doi.org/10.1016/j.neulet.2006.10.063
Bohlig L, Rother K (2011) One function—multiple mechanisms: the manifold activities of p53 as a transcriptional repressor. J Biomed Biotechnol 2011:464916–464915. https://doi.org/10.1155/2011/464916
Volonte C, Apolloni S, Parisi C (2015) MicroRNAs: newcomers into the ALS picture. CNS Neurol Disord Drug Targets 14(2):194–207
Ma X, Becker Buscaglia LE, Barker JR, Li Y (2011) MicroRNAs in NF-kappaB signaling. J Mol Cell Biol 3(3):159–166. https://doi.org/10.1093/jmcb/mjr007
Iyer A, Zurolo E, Prabowo A, Fluiter K, Spliet WG, van Rijen PC, Gorter JA, Aronica E (2012) MicroRNA-146a: a key regulator of astrocyte-mediated inflammatory response. PLoS One 7(9):e44789. https://doi.org/10.1371/journal.pone.0044789
Bunton-Stasyshyn RK, Saccon RA, Fratta P, Fisher EM (2015) SOD1 function and its implications for amyotrophic lateral sclerosis pathology: new and renascent themes. Neuroscientist 21(5):519–529. https://doi.org/10.1177/1073858414561795
Ferraiuolo L, Meyer K, Sherwood TW, Vick J, Likhite S, Frakes A, Miranda CJ, Braun L et al (2016) Oligodendrocytes contribute to motor neuron death in ALS via SOD1-dependent mechanism. Proc Natl Acad Sci U S A 113(42):E6496–E6505. https://doi.org/10.1073/pnas.1607496113
Philips T, Bento-Abreu A, Nonneman A, Haeck W, Staats K, Geelen V, Hersmus N, Kusters B et al (2013) Oligodendrocyte dysfunction in the pathogenesis of amyotrophic lateral sclerosis. Brain 136(Pt 2):471–482. https://doi.org/10.1093/brain/aws339
Nikodemova M, Small AL, Smith SMC, Mitchell GS, Watters JJ (2014) Spinal but not cortical microglia acquire an atypical phenotype with high VEGF, galectin-3 and osteopontin, and blunted inflammatory responses in ALS rats23. Neurobiol Dis 69:43–53. https://doi.org/10.1016/j.nbd.2013.11.009
Cardoso AL, Guedes JR, Pereira de Almeida L, Pedroso de Lima MC (2012) miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology 135(1):73–88. https://doi.org/10.1111/j.1365-2567.2011.03514.x
Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, Song S, Likhite S et al (2011) Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol 29(9):824–828. https://doi.org/10.1038/nbt.1957
Theodoric N, Bechberger JF, Naus CC, Sin WC (2012) Role of gap junction protein connexin43 in astrogliosis induced by brain injury. PLoS One 7(10):e47311. https://doi.org/10.1371/journal.pone.0047311
Serrano A, Donno C, Giannetti S, Peric M, Andjus P, D'Ambrosi N, Michetti F (2017) The astrocytic S100B protein with its receptor RAGE is aberrantly expressed in SOD1(G93A) models, and its inhibition decreases the expression of proinflammatory genes. Mediat Inflamm 2017:1626204. https://doi.org/10.1155/2017/1626204
Tian R, Wu X, Hagemann TL, Sosunov AA, Messing A, McKhann GM, Goldman JE (2010) Alexander disease mutant glial fibrillary acidic protein compromises glutamate transport in astrocytes. J Neuropathol Exp Neurol 69(4):335–345. https://doi.org/10.1097/NEN.0b013e3181d3cb52
Lepekhin EA, Eliasson C, Berthold CH, Berezin V, Bock E, Pekny M (2001) Intermediate filaments regulate astrocyte motility. J Neurochem 79(3):617–625
Yoshii Y, Otomo A, Pan L, Ohtsuka M, Hadano S (2011) Loss of glial fibrillary acidic protein marginally accelerates disease progression in a SOD1(H46R) transgenic mouse model of ALS. Neurosci Res 70(3):321–329. https://doi.org/10.1016/j.neures.2011.03.006
Baker DJ, Blackburn DJ, Keatinge M, Sokhi D, Viskaitis P, Heath PR, Ferraiuolo L, Kirby J et al (2015) Lysosomal and phagocytic activity is increased in astrocytes during disease progression in the SOD1 (G93A) mouse model of amyotrophic lateral sclerosis. Front Cell Neurosci 9:410. https://doi.org/10.3389/fncel.2015.00410
Sidoryk-Wegrzynowicz M, Gerber YN, Ries M, Sastre M, Tolkovsky AM, Spillantini MG (2017) Astrocytes in mouse models of tauopathies acquire early deficits and lose neurosupportive functions. Acta Neuropathol Commun 5(1):89. https://doi.org/10.1186/s40478-017-0478-9
Li M, Sun L, Luo Y, Xie C, Pang Y, Li Y (2014) High-mobility group box 1 released from astrocytes promotes the proliferation of cultured neural stem/progenitor cells. Int J Mol Med 34(3):705–714. https://doi.org/10.3892/ijmm.2014.1820
Xie ZF, Xin G, Xu YX, Su Y, Li KS (2016) LPS-primed release of HMGB-1 from cortical astrocytes is modulated through PI3K/AKT pathway. Cell Mol Neurobiol 36(1):93–102. https://doi.org/10.1007/s10571-015-0223-5
Pedrazzi M, Patrone M, Passalacqua M, Ranzato E, Colamassaro D, Sparatore B, Pontremoli S, Melloni E (2007) Selective proinflammatory activation of astrocytes by high-mobility group box 1 protein signaling. J Immunol 179(12):8525–8532
Bhalala OG, Pan L, Sahni V, McGuire TL, Gruner K, Tourtellotte WG, Kessler JA (2012) microRNA-21 regulates astrocytic response following spinal cord injury. J Neurosci 32(50):17935–17947. https://doi.org/10.1523/JNEUROSCI.3860-12.2012
Strickland ER, Hook MA, Balaraman S, Huie JR, Grau JW, Miranda RC (2011) MicroRNA dysregulation following spinal cord contusion: implications for neural plasticity and repair. Neuroscience 186:146–160. https://doi.org/10.1016/j.neuroscience.2011.03.063
Qian K, Huang H, Peterson A, Hu B, Maragakis NJ, Ming GL, Chen H, Zhang SC (2017) Sporadic ALS astrocytes induce neuronal degeneration in vivo. Stem Cell Reports 8(4):843–855. https://doi.org/10.1016/j.stemcr.2017.03.003
Tripathi P, Rodriguez-Muela N, Klim JR, de Boer AS, Agrawal S, Sandoe J, Lopes CS, Ogliari KS et al (2017) Reactive astrocytes promote ALS-like degeneration and intracellular protein aggregation in human motor neurons by disrupting autophagy through TGF-beta1. Stem Cell Reports 9(2):667–680. https://doi.org/10.1016/j.stemcr.2017.06.008
Casas C, Manzano R, Vaz AR, Rosario O, Brites D (2016) Synaptic failure: focus in an integrative view of ALS. Brain Plast 1(2):159–175
Thomsen GM, Gowing G, Latter J, Chen M, Vit JP, Staggenborg K, Avalos P, Alkaslasi M et al (2014) Delayed disease onset and extended survival in the SOD1G93A rat model of amyotrophic lateral sclerosis after suppression of mutant SOD1 in the motor cortex. J Neurosci 34(47):15587–15600. https://doi.org/10.1523/JNEUROSCI.2037-14.2014
Wang J, Xu G, Borchelt DR (2002) High molecular weight complexes of mutant superoxide dismutase 1: age-dependent and tissue-specific accumulation. Neurobiol Dis 9(2):139–148. https://doi.org/10.1006/nbdi.2001.0471
Wang M, Xu Q, Yuan M (2011) The unfolded protein response induced by salt stress in Arabidopsis. Methods Enzymol 489:319–328. https://doi.org/10.1016/B978-0-12-385116-1.00018-2
Saxena S, Cabuy E, Caroni P (2009) A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat Neurosci 12(5):627–636. https://doi.org/10.1038/nn.2297
Yip PK, Pizzasegola C, Gladman S, Biggio ML, Marino M, Jayasinghe M, Ullah F, Dyall SC et al (2013) The omega-3 fatty acid eicosapentaenoic acid accelerates disease progression in a model of amyotrophic lateral sclerosis. PLoS One 8(4):e61626. https://doi.org/10.1371/journal.pone.0061626
Genc B, Jara JH, Lagrimas AK, Pytel P, Roos RP, Mesulam MM, Geula C, Bigio EH et al (2017) Apical dendrite degeneration, a novel cellular pathology for Betz cells in ALS. Sci Rep 7:41765. https://doi.org/10.1038/srep41765
Cruz-Sanchez FF, Moral A, Rossi ML, Quinto L, Castejon C, Tolosa E, de Belleroche J (1996) Synaptophysin in spinal anterior horn in aging and ALS: an immunohistological study. J Neural Transm (Vienna) 103(11):1317–1329. https://doi.org/10.1007/BF01271192
Abe M, Bonini NM (2013) MicroRNAs and neurodegeneration: role and impact. Trends Cell Biol 23(1):30–36. https://doi.org/10.1016/j.tcb.2012.08.013
Zeng Y (2009) Regulation of the mammalian nervous system by microRNAs. Mol Pharmacol 75(2):259–264. https://doi.org/10.1124/mol.108.052118
Quiroz JFD, Tsai E, Coyle M, Sehm T, Echeverri K (2014) Precise control of miR-125b levels is required to create a regeneration-permissive environment after spinal cord injury: a cross-species comparison between salamander and rat. Dis Model Mech 7(6):601–611. https://doi.org/10.1242/dmm.014837
Zhou F, Zhang C, Guan Y, Chen Y, Lu Q, Jie L, Gao H, Du H et al (2017) Screening the expression characteristics of several miRNAs in G93A-SOD1 transgenic mouse: altered expression of miRNA-124 is associated with astrocyte differentiation by targeting Sox2 and Sox9. J Neurochem. https://doi.org/10.1111/jnc.14229
Campos-Melo D, Droppelmann CA, He Z, Volkening K, Strong MJ (2013) Altered microRNA expression profile in amyotrophic lateral sclerosis: a role in the regulation of NFL mRNA levels. Mol Brain 6:26. https://doi.org/10.1186/1756-6606-6-26
Benigni M, Ricci C, Jones AR, Giannini F, Al-Chalabi A, Battistini S (2016) Identification of miRNAs as potential biomarkers in cerebrospinal fluid from amyotrophic lateral sclerosis patients. NeuroMolecular Med 18(4):551–560. https://doi.org/10.1007/s12017-016-8396-8
Casula M, Iyer AM, Spliet WG, Anink JJ, Steentjes K, Sta M, Troost D, Aronica E (2011) Toll-like receptor signaling in amyotrophic lateral sclerosis spinal cord tissue. Neuroscience 179:233–243. https://doi.org/10.1016/j.neuroscience.2011.02.001
Crosio C, Valle C, Casciati A, Iaccarino C, Carri MT (2011) Astroglial inhibition of NF-kappaB does not ameliorate disease onset and progression in a mouse model for amyotrophic lateral sclerosis (ALS). PLoS One 6(3):e17187. https://doi.org/10.1371/journal.pone.0017187
Liu Z, Li Y, Cui Y, Roberts C, Lu M, Wilhelmsson U, Pekny M, Chopp M (2014) Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia 62(12):2022–2033. https://doi.org/10.1002/glia.22723
Kunze A, Lengacher S, Dirren E, Aebischer P, Magistretti PJ, Renaud P (2013) Astrocyte-neuron co-culture on microchips based on the model of SOD mutation to mimic ALS. Integr Biol (Camb) 5(7):964–975. https://doi.org/10.1039/c3ib40022k
Foran E, Trotti D (2009) Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis. Antioxid Redox Signal 11(7):1587–1602. https://doi.org/10.1089/ars.2009.2444
Mei J, Bachoo R, Zhang CL (2011) MicroRNA-146a inhibits glioma development by targeting Notch1. Mol Cell Biol 31(17):3584–3592. https://doi.org/10.1128/MCB.05821-11
Zhang QB, Qing YF, Yin CC, Zhou L, Liu XS, Mi QS, Zhou JG (2018) Mice with miR-146a deficiency develop severe gouty arthritis via dysregulation of TRAF 6, IRAK 1 and NALP3 inflammasome. Arthritis Res Ther 20(1):45. https://doi.org/10.1186/s13075-018-1546-7
Taganov KD, Boldin MP, Chang KJ, Baltimore D (2006) NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 103(33):12481–12486. https://doi.org/10.1073/pnas.0605298103
Lakatos A (2017) State-of-art modelling of inflammatory astrocyte-synapse interactions in injury and amyotrophic lateral sclerosis. Neural Regen Res 12(1):75–76. https://doi.org/10.4103/1673-5374.198977
Kaplan A, Spiller KJ, Towne C, Kanning KC, Choe GT, Geber A, Akay T, Aebischer P et al (2014) Neuronal matrix metalloproteinase-9 is a determinant of selective neurodegeneration. Neuron 81(2):333–348. https://doi.org/10.1016/j.neuron.2013.12.009
Fang P, Schachner M, Shen YQ (2012) HMGB1 in development and diseases of the central nervous system. Mol Neurobiol 45(3):499–506. https://doi.org/10.1007/s12035-012-8264-y
Acknowledgments
CG and CC are recipients of PhD fellowships from FCT (SFRH/BD/102718/2014 and SFRH/BD/91316/2012, respectively) and ARV of a Postdoctoral grant (SFRH/BPD/76590/2011). The funding organization had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Dr. Andreia Barateiro and Dr. Ana Domingos for acquisition of brain section images in Leica DM6000 inverted Confocal Microscope at Instituto Gulbenkian de Ciência (Oeiras, Portugal).
Funding
This work was supported by the Research Grant of the Santa Casa Scientific Research Program on ALS, by Santa Casa da Misericórdia de Lisboa (SCML), Portugal, Project Ref. ELA-2015-002 (to DB), by the project PTDC/SAU-FAR/118787/2010 (to DB) and, in part, by iMed.ULisboa (UID/DTP/04138/2013) from Fundação para a Ciência e a Tecnologia (FCT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was performed in accordance with the European Community guidelines (Directives 86/609/EU and 2010/63/EU, Recommendation 2007/526/CE, European Convention for the Protection of Vertebrate Animals used for Experimental or Other Scientific Purposes ETS 123/Appendix A) and Portuguese Laws on Animal Care (Decreto-Lei 129/92, Portaria 1005/92, Portaria 466/95, Decreto-Lei 197/96, Portaria 1131/97). All the protocols used in this study were approved by the Portuguese National Authority (General Direction of Veterinary) and the Ethics Committee of the Instituto de Medicina Molecular (iMM) of the Faculty of Medicine, Universidade de Lisboa, Lisbon, Portugal.
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Figure 1
Unchanged LC3B and Beclin-1 protein expression in the cerebral cortex of mSOD1 mice indicate that the autophagy process is not affected by the disease. Cortical tissue samples were obtained from hSOD1G93A (mSOD1) and wild-type (WT) mice at 4–6 week-old (pre-symptomatic, stage 1) and 12–14 week-old (symptomatic, stage 2). Western blot analysis showing the expression levels of (a) microtubule-associated protein 1 light chain 3B (LC3B) and (b) Beclin-1. Representative results from one blot are shown. β-actin was used as a loading control. Results are represented as fold vs. WT animals in the same disease stage. Data represent mean values ± SEM from at least five independent experiments. (PNG 219 kb)
Supplementary Figure 2
Expression levels of proteins associated with oligodendrocyte progenitors and activated microglia in the cerebral cortex of mSOD1 mice are not affected by the disease, though the population of mature myelinating oligodendrocytes is reduced before disease onset. . Cortical tissue samples were obtained from hSOD1G93A (mSOD1) and wild-type (WT) mice at 4–6 week-old (pre-symptomatic, stage 1) and 12–14 week-old (symptomatic, stage 2). Western blot analysis showing the expression levels of (a) NG2 and (c) myelin basic protein (MBP). Representative results from one blot are shown. β-actin was used as a loading control. qRT-PCR gene expression analysis of (b) NG2, (d) CD11b mRNA and (e) microRNA(miR)-155 levels. Results are represented as folds vs. age-matched WT mice. Data represent mean values ± SEM from at least five independent experiments. *p < 0.05 vs. WT samples, two-tailed unpaired Student’s t-test with Welch’s correction when required. (PNG 372 kb)
Supplementary Figure 3
Astrocytes isolated from the cerebral cortex of mSOD1 mice show a predominance of cells globally presenting an atypical reactive phenotype. Astrocytes were isolated from the cortex of hSOD1G93A (mSOD1) and wild-type (WT) mice pups at 7 day-old, and cultured for 13 days in vitro. Representative fluorescence images of S100 calcium-binding protein B (S100B), the proliferation marker Ki-67, fibrillary acidic protein (GFAP), glutamate transporter 1 (GLT-1), glutamate aspartate transporter (GLAST) and, vimentin and Respective quantification based on the number of positive cells above a fluorescent signal threshold settled for each marker. For Ki-67 staining, only cells with 2 or more nuclear punctuations were considered as positive. Results are represented as folds vs. WT samples. Data represent mean values ± SEM from at least five independent experiments. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. WT samples, two-tailed unpaired Student’s t-test with Welch’s correction when required. Scale bar represents 40 μm. (PNG 2150 kb)
Supplementary Table 1
(DOCX 17 kb)
Supplementary Table 2
(DOCX 20.3 kb)
Supplementary Table 3
(DOCX 18 kb)
Supplementary Table 4
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Gomes, C., Cunha, C., Nascimento, F. et al. Cortical Neurotoxic Astrocytes with Early ALS Pathology and miR-146a Deficit Replicate Gliosis Markers of Symptomatic SOD1G93A Mouse Model. Mol Neurobiol 56, 2137–2158 (2019). https://doi.org/10.1007/s12035-018-1220-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1220-8