Abstract
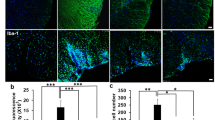
Recent studies showed that neuronal surface protein CD200 plays a key role in the regulation of neuroinflammation. Previously, we showed that arsenic (0.38 mg/kg body weight) exposure induces microglial activation and consequently IL-6/TNF-α secretion. This result indicated the possibility of alteration in the expression of CD200. Therefore, the present study was focused on checking arsenic-induced alteration in CD200 expression and revealing the underlying mechanism. Male BALB/c mice were exposed to arsenic (vehicle, 0.038 and 0.38 mg/kg body weight) for 60 days, and the expression level of CD200 was found to be decreased which was rescued by minocycline (33 mg/kg body weight) co-administration. Higher CD68 staining, increased level of IL-6/TNF-α, as well as higher level of IFNγ, were observed in in vivo arsenic-exposed groups. Interestingly, in vitro arsenic exposure could not increase IL-6/TNF-α level in the culture supernatant, whereas, supplementation of IFNγ could mimic the in vivo results. However, arsenic could not induce IFNγ production from brain endothelial cells, microglia, and astrocytes, thereby suggesting the entry of IFNγ through the impaired blood–brain barrier. Evans blue fluorescence in the brain confirms altered blood–brain barrier permeability although no changes were observed in the expression level of tight junction proteins (claudin-5 and occludin). Finally, intracerebral injection of anti-IFNγ neutralizing antibody in arsenic-exposed brain reduced microglia activation (IL-6 and TNF-α and CD68 expression) and subsequently rescued CD200 level. Taken together, the study showed that arsenic-mediated compromised blood–brain barrier is a major driving force to induce microglial IL-6 and TNF-α production through serum IFNγ leading to CD200 downregulation.








Similar content being viewed by others
Change history
02 August 2018
The original version of this article unfortunately contained mistake on Fig. 3A.
References
Dentesano G, Serratosa J, Tusell JM, Ramón P, Valente T, Saura J, Solà C (2014) CD200R1 and CD200 expression are regulated by PPAR-γ in activated glial cells. Glia 62(6):982–998
Walker DG, Dalsing-Hernandez JE, Campbell NA, Lue L-F (2009) Decreased expression of CD200 and CD200 receptor in Alzheimer’s disease: a potential mechanism leading to chronic inflammation. Exp Neurol 215(1):5–19
Zhang S, Wang X-J, Tian L-P, Pan J, Lu G-Q, Zhang Y-J, Ding J-Q, Chen S-D (2011) CD200-CD200R dysfunction exacerbates microglial activation and dopaminergic neurodegeneration in a rat model of Parkinson’s disease. J Neuroinflammation 8(1):154. https://doi.org/10.1186/1742-2094-8-154
Sun F-J, Zhang C-Q, Chen X, Wei Y-J, Li S, Liu S-Y, He J-J, Guo W et al (2016) Downregulation of CD47 and CD200 in patients with focal cortical dysplasia type IIb and tuberous sclerosis complex. J Neuroinflammation 13(1):85
Denieffe S, Kelly RJ, McDonald C, Lyons A, Lynch MA (2013) Classical activation of microglia in CD200-deficient mice is a consequence of blood brain barrier permeability and infiltration of peripheral cells. Brain Behav Immun 34:86–97
Chitnis T, Imitola J, Wang Y, Elyaman W, Chawla P, Sharuk M, Raddassi K, Bronson RT et al (2007) Elevated neuronal expression of CD200 protects Wld s mice from inflammation-mediated neurodegeneration. Am J Pathol 170(5):1695–1712
Cox FF, Carney D, Miller A-M, Lynch MA (2012) CD200 fusion protein decreases microglial activation in the hippocampus of aged rats. Brain Behav Immun 26(5):789–796
Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, Lynch MA (2007) CD200 ligand–receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci 27(31):8309–8313
Lyons A, McQuillan K, Deighan BF, O’Reilly J-A, Downer EJ, Murphy AC, Watson M, Piazza A et al (2009) Decreased neuronal CD200 expression in IL-4-deficient mice results in increased neuroinflammation in response to lipopolysaccharide. Brain Behav Immun 23(7):1020–1027
Rosenblum MD, Olasz E, Woodliff JE, Johnson BD, Konkol MC, Gerber KA, Orentas RJ, Sandford G et al (2004) CD200 is a novel p53-target gene involved in apoptosis-associated immune tolerance. Blood 103(7):2691–2698
Chen Z, Marsden PA, Gorczynski RM (2006) Cloning and characterization of the human CD200 promoter region. Mol Immunol 43(6):579–587
Singh V, Gera R, Kushwaha R, Sharma AK, Patnaik S, Ghosh D (2016) Hijacking microglial glutathione by inorganic arsenic impels bystander death of immature neurons through extracellular cystine/glutamate imbalance. Sci Rep 6:30601
Gera R, Singh V, Mitra S, Sharma AK, Singh A, Dasgupta A, Singh D, Kumar M et al (2017) Arsenic exposure impels CD4 commitment in thymus and suppress T cell cytokine secretion by increasing regulatory T cells. Sci Rep 7(1):7140
Rai A, Maurya SK, Khare P, Srivastava A, Bandyopadhyay S (2010) Characterization of developmental neurotoxicity of As, Cd, and Pb mixture: Synergistic action of metal mixture in glial and neuronal functions. Toxicol Sci 118(2):586–601
Singh V, Mitra S, Sharma AK, Gera R, Ghosh D (2014) Isolation and characterization of microglia from adult mouse brain: selected applications for ex vivo evaluation of immunotoxicological alterations following in vivo xenobiotic exposure. Chem Res Toxicol 27(5):895–903
Tyler CR, Allan AM (2014) The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr Environ Health Rep 1(2):132–147
Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H et al (2013) Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis 4(3):e525
Saura J, Tusell JM, Serratosa J (2003) High-yield isolation of murine microglia by mild trypsinization. Glia 44(3):183–189
Pardo B, Contreras L, Serrano A, Ramos M, Kobayashi K, Iijima M, Saheki T, Satrústegui J (2006) Essential role of aralar in the transduction of small Ca+ signals to neuronal mitochondria. J Biol Chem 281(2):1039–1047
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1):248–254
Gasche Y, Copin J-C, Sugawara T, Fujimura M, Chan PH (2001) Matrix metalloproteinase inhibition prevents oxidative stress-associated blood–brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab 21(12):1393–1400
Paxinos G (2013) Paxinos and Franklin’s the mouse brain in stereotaxic coordinates. Elsevier/Academic Press, Boston
Colaianna M, Tucci P, Zotti M, Morgese M, Schiavone S, Govoni S, Cuomo V, Trabace L (2010) Soluble βamyloid1-42: a critical player in producing behavioural and biochemical changes evoking depressive-related state? Br J Pharmacol 159(8):1704–1715
Gharibzadeh S, Hoseini SS (2008) Arsenic exposure may be a risk factor for Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 20(4):501–501
Ashok A, Rai NK, Tripathi S, Bandyopadhyay S (2014) Exposure to As-, Cd-, and Pb-mixture induces Aβ, amyloidogenic APP processing and cognitive impairments via oxidative stress-dependent neuroinflammation in young rats. Toxicol Sci 143(1):64–80
Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14(4):388–405
Liu M-C, Liu X-Q, Wang W, Shen X-F, Che H-L, Guo Y-Y, Zhao M-G, Chen J-Y et al (2012) Involvement of microglia activation in the lead induced long-term potentiation impairment. PLoS One 7(8):e43924
Zhao F, Cai T, Liu M, Zheng G, Luo W, Chen J (2008) Manganese induces dopaminergic neurodegeneration via microglial activation in a rat model of manganism. Toxicol Sci 107(1):156–164
Kauppinen TM, Higashi Y, Suh SW, Escartin C, Nagasawa K, Swanson RA (2008) Zinc triggers microglial activation. J Neurosci 28(22):5827–5835
Mishra MK, Basu A (2008) Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis. J Neurochem 105(5):1582–1595
Pan W, Banks WA, Kastin AJ (1997) Permeability of the blood–brain and blood–spinal cord barriers to interferons. J Neuroimmunol 76(1):105–111
Sherwood CL, Liguori AE, Olsen CE, Lantz RC, Burgess JL, Boitano S (2013) Arsenic compromises conducting airway epithelial barrier properties in primary mouse and immortalized human cell cultures. PLoS One 8(12):e82970
Cheng T-J, Ke D-S, Guo H-R (2010) The association between arsenic exposure from drinking water and cerebrovascular disease mortality in Taiwan. Water Res 44(19):5770–5776
Funding
This work was supported by CSIR-12th 5-year network project, INDEPTH (BSC001) (CSIR, Council of Scientific and Industrial Research). V.S and J.M were supported by CSIR Senior Research Fellowship. R.G, J.D, and J.A.A were supported by UGC-Senior Research Fellowship, respectively. S.K was supported by CSIR Junior Research Fellowship. The CSIR-IITR manuscript number is 3528.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Singh, V., Kushwaha, S., Gera, R. et al. Sneaky Entry of IFNγ Through Arsenic-Induced Leaky Blood–Brain Barrier Reduces CD200 Expression by Microglial pro-Inflammatory Cytokine. Mol Neurobiol 56, 1488–1499 (2019). https://doi.org/10.1007/s12035-018-1155-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1155-0