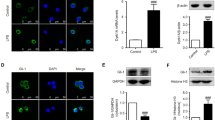
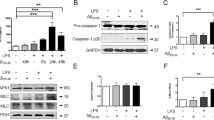
Abstract
Down syndrome is characterized by premature aging and dementia with neurological features that mimic those found in Alzheimer’s disease. This pathology in Down syndrome could be related to inflammation, which plays a role in other neurodegenerative diseases. We previously found a link between the NFkB pathway, long considered a prototypical proinflammatory signaling pathway, and the dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A). DYRK1A is associated with early onset of Alzheimer’s disease in Down syndrome patients. Here, we sought to determine the role of DYRK1A on regulation of the NFkB pathway in the mouse brain. We found that over-expression of Dyrk1A (on a C57BL/6J background) stabilizes IκBα protein levels by inhibition of calpain activity and increases cytoplasmic p65 sequestration in the mouse brain. In contrast, Dyrk1A-deficient mice (on a CD1 background) have decreased IκBα protein levels with an increased calpain activity and decreased cytoplasmic p65 sequestration in the brain. Taken together, our results demonstrate a role of DYRK1A in regulation of the NFkB pathway. However, decreased IκBα and DYRK1A protein levels associated with an increased calpain activity were found in the brains of mice over-expressing Dyrk1A after lipopolysaccharide treatment. Although inflammation induced by lipopolysaccharide treatment has a positive effect on calpastatin and a negative effect on DYRK1A protein level, a positive effect on microglial activation is maintained in the brains of mice over-expressing Dyrk1A.






Similar content being viewed by others
References
Agostinho P, Cunha RA, Oliveira C (2010) Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr Pharm Des 16:2766–2778
Lue LF, Kuo YM, Beach T, Walker DG (2010) Microglia activation and anti-inflammatory regulation in Alzheimer’s disease. Mol Neurobiol 41:115–128
Baeuerle PA, Henkel T (1994) Function and activation of NF-kappa B in the immune system. Annu Rev Immunol 12:141–179
Marwarha G, Ghribi O (2017) Nuclear factor Kappa-light-chain-enhancer of activated B cells (NF-κB)—a friend, a foe, or a bystander—in the neurodegenerative cascade and pathogenesis of Alzheimer’s disease. CNS Neurol Disord Drug Targets 16:1050–1065
Jones SV, Kounatidis I (2017) Nuclear factor-kappa B and Alzheimer disease, unifying genetic and environmental risk factors from cell to humans. Front Immunol 8:1805
Kaltschmidt B, Kaltschmidt C (2009) NF-kappaB in the nervous system. Cold Spring Harb Perspect Biol 1:a001271
Hoffmann A, Baltimore D (2006) Circuitry of nuclear factor kappaB signaling. Immunol Rev 210:171–186
DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M (1997) A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature 388:548–554
Schaecher K, Goust JM, Banik NL (2004) The effects of calpain inhibition on IkB alpha degradation after activation of PBMCs: identification of the calpain cleavage sites. Neurochem Res 29:1443–1451
Han Y, Weinman S, Boldogh I, Walker RK, Brasier AR (1999) Tumor necrosis factor-alpha-inducible IkappaBalpha proteolysis mediated by cytosolic m-calpain. A mechanism parallel to the ubiquitin-proteasome pathway for nuclear factor-kappab activation. J Biol Chem 274:787–794
Shumway SD, Maki M, Miyamoto S (1999) The PEST domain of IkappaBalpha is necessary and sufficient for in vitro degradation by mu-calpain. J Biol Chem 274:30874–30881
Camins A, Verdaguer E, Folch J, Pallàs M (2006) Involvement of calpain activation in neurodegenerative processes. CNS Drug Rev 12:135–148
Liu SH, Yang CN, Pan HC, Sung YJ, Liao KK, Chen WB, Lin WZ, Sheu ML (2010) IL-13 downregulates PPAR-gamma/heme oxygenase-1 via ER stress-stimulated calpain activation: aggravation of activated microglia death. Cell Mol Life Sci 67:1465–1476
Granese B, Scala I, Spatuzza C, Valentino A, Coletta M, Vacca RA, De Luca P, Andria G (2013) Validation of microarray data in human lymphoblasts shows a role of the ubiquitin-proteasome system and NF-kB in the pathogenesis of Down syndrome. BMC Med Genet 6:24
Ryoo SR, Cho HJ, Lee HW, Jeong HK, Radnaabazar C, Kim YS, Kim MJ, Son MY et al (2008) Dual-specificity tyrosine(Y)-phosphorylation regulated kinase 1A-mediated phosphorylation of amyloid precursor protein: evidence for a functional link between down syndrome and Alzheimer’s disease. J Neurochem 104:1333–1344
Park J, Oh Y, Chung KC (2009) Two key genes closely implicated with the neuropathological characteristics in Down syndrome: DYRK1A and RCAN1. BMB Rep 42:6–15
Noll C, Tlili A, Ripoll C, Mallet L, Paul JL, Delabar JM, Janel N (2012) Dyrk1a activates antioxidant NQO1 expression through an ERK1/2-Nrf2 dependent mechanism. Mol Genet Metab 105:484–488
Guedj F, Pereira PL, Najas S, Barallobre MJ, Chabert C, Souchet B, Sebrie C, Verney C et al (2012) DYRK1A: a master regulatory protein controlling brain growth. Neurobiol Dis 46:190–203
Li Z, Yu T, Morishima M, Pao A, LaDuca J, Conroy J, Nowak N, Matsui S et al (2007) Duplication of the entire 22.9 Mb human chromosome 21 syntenic region on mouse chromosome 16 causes cardiovascular and gastrointestinal abnormalities. Hum Mol Genet 16:1359–1366
Fotaki V, Dierssen M, Alcantara S, Martinez S, Marti E, Casas C, Visa J, Soriano E et al (2002) Dyrk1A haploin- sufficiency affects viability and causes developmental delay and abnormal brain morphology in mice. Mol Cell Biol 22:6636–6647
Ducros V, Demuth K, Sauvant MP, Quillard M, Caussé E, Candito M, Read MH, Drai J et al (2002) Methods for homocysteine analysis and biological relevance of the results. J Chromatogr B Analyt Technol Biomed Life Sci 781:207–226
Grintal B, Champeil-Potokar G, Lavialle M, Vancassel S, Breton S, Denis I (2009) Inhibition of astroglial glutamate transport by polyunsaturated fatty acids: evidence for a signalling role of docosahexaenoic acid. Neurochem Int 54:535–543
Kohli V, Gao W, Camargo CA, Clavien PA (1997) Calpain is a mediator of preservation-reperfusion injury in rat liver transplantation. Proc Natl Acad Sci U S A 94:9354–9359
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36
Chen D, Li X, Zhai Z, Shu HB (2002) A novel zinc finger protein interacts with receptor-interacting protein (RIP) and inhibits tumor necrosis factor (TNF)- and IL1-induced NF-kappa B activation. J Biol Chem 277:15985–911598
Oeckinghaus A, Ghosh S (2009) The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 1:a000034
Kaltschmidt B, Widera D, Kaltschmidt C (2005) Signaling via NF-kappaB in the nervous system. Biochim Biophys Acta 1745:287–299
Hamakubo T, Kannagi R, Murachi T, Matus A (1986) Distribution of calpains I and II in rat brain. J Neurosci 6:3103–3111
Santos DM, Xavier JM, Morgado AL, Solá S, Rodrigues CM (2012) Distinct regulatory functions of calpain 1 and 2 during neural stem cell self-renewal and differentiation. PLoS One 7:e33468
Noll C, Planque C, Ripoll C, Guedj F, Diez A, Ducros V, Belin N, Duchon A et al (2009) DYRK1A, a novel determinant of the methionine-homocysteine cycle in different mouse models overexpressing this Down-syndrome-associated kinase. PLoS One 4:e7540
Wang WY, Tan MS, Yu JT, Tan L (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med 3:136
Kim YS1, Cho KO, Lee HJ, Kim SY, Sato Y, Cho YJ (2006) Down syndrome candidate region 1 increases the stability of the IkappaBalpha protein: implications for its anti-inflammatory effects. J Biol Chem 281:39051–39061
Pahl HL (1999) Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18:6853–6666
Jazwa A, Cuadrado A (2010) Targeting heme oxygenase-1 for neuroprotection and neuroinflammation in neurodegenerative diseases. Curr Drug Targets 11:1517–1531
Zocchia C, Spiga G, Rabin SJ, Grekova M, Richert J, Chernyshev O, Colton C, Mocchetti I (1997) Biological activity of interleukin-10 in the central nervous system. Neurochem Int 30:433–439
Mosser DM, Zhang X (2008) Interleukin-10: new perspectives on an old cytokine. Immunol Rev 226:205–218
Kaltschmidt C, Kaltschmidt B, Baeuerle PA (1995) Stimulation of ionotropic glutamate receptors activates transcription factor NF-kappa B in primary neurons. Proc Natl Acad Sci U S A 92:9618–9622
Lilienbaum A, Israël A (2003) From calcium to NF-kappa B signaling pathways in neurons. Mol Cell Biol 23:2680–2698
Souchet B, Guedj F, Sahún I, Duchon A, Daubigney F, Badel A, Yanagawa Y, Barallobre MJ et al (2014) Excitation/inhibition balance and learning are modified by Dyrk1a gene dosage. Neurobiol Dis 69:65–75
Schölzke MN, Potrovita I, Subramaniam S, Prinz S, Schwaninger M (2003) Glutamate activates NF-kappaB through calpain in neurons. Eur J Neurosci 18:3305–3310
James P, Vorherr T, Krebs J, Morelli A, Castello G, McCormick DJ, Penniston JT, De Flora A et al (1989) Modulation of erythrocyte Ca2+-ATPase by selective calpain cleavage of the calmodulin-binding domain. J Biol Chem 264:8289–8296
Wang Y, Briz V, Chishti A, Bi X, Baudry M (2013) Distinct roles for μ-calpain and m-calpain in synaptic NMDAR-mediated neuroprotection and extrasynaptic NMDAR-mediated neurodegeneration. J Neurosci 33:18880–18892
Baudry M, Bi X (2016) Calpain-1 and calpain-2: the yin and yang of synaptic plasticity and neurodegeneration. Trends Neurosci 39:235–245
Aguzzi A, Barres BA, Bennett ML (2013) Microglia: scapegoat, saboteur, or something else? Science 339:156–161
Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR 3rd, Lafaille JJ, Hempstead BL, Littman DR et al (2013) Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155:1596–1609
Zadran S, Jourdi H, Rostamiani K, Qin Q, Bi X, Baudry M (2010) Brain-derived neurotrophic factor and epidermal growth factor activate neuronal m-calpain via mitogen-activated protein kinase-dependent phosphorylation. J Neurosci 30:1086–1095
Guedj F, Sébrié C, Rivals I, Ledru A, Paly E, Bizot JC, Smith D, Rubin E et al (2009) Green tea polyphenols rescue of brain defects induced by overexpression of DYRK1A. PLoS One 4:e4606
Toiber D, Azkona G, Ben-Ari S, Torán N, Soreq H, Dierssen M (2010) Engineering DYRK1A overdosage yields Down syndrome-characteristic cortical splicing aberrations. Neurobiol Dis 40:348–359
Dohi K, Ohtaki H, Nakamachi T, Yofu S, Satoh K, Miyamoto K, Song D, Tsunawaki S et al (2010) Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J Neuroinflammation 7:41
Mattson MP, Culmsee C, Yu Z, Camandola S (2000) Roles of nuclear factor kappaB in neuronal survival and plasticity. J Neurochem 74:443–456
LaFerla FM (2002) Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat Rev Neurosci 3:862–872
Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D (2003) NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci 6:1072–1078
Bartus R (1997) The calpain hypothesis of neurodegeneration: evidence for a common cytotoxic pathway. Neuroscientist 3:314–327
Rao MV, Mohan PS, Peterhoff CM, Yang DS, Schmidt SD, Stavrides PH, Campbell J, Chen Y et al (2008) Marked calpastatin (CAST) depletion in Alzheimer’s disease accelerates cytoskeleton disruption and neurodegeneration: neuroprotection by CAST overexpression. J Neurosci 28:12241–12254
Goñi-Oliver P, Lucas JJ, Avila J, Hernández F (2007) N-terminal cleavage of GSK-3 by calpain: a new form of GSK-3 regulation. J Biol Chem 282:22406–22413
Song WJ, Song EA, Jung MS, Choi SH, Baik HH, Jin BK, Kim JH, Chung SH (2015) Phosphorylation and inactivation of glycogen synthase kinase 3β (GSK3β) by dual-specificity tyrosine phosphorylation-regulated kinase 1A (Dyrk1A). J Biol Chem 290:2321–2333
Hamelet J, Noll C, Ripoll C, Paul JL, Janel N, Delabar JM (2009) Effect of hyperhomocysteinemia on the protein kinase DYRK1A in liver of mice. Biochem Biophys Res Commun 378:673–677
Cheng Z, Jiang X, Pansuria M, Fang P, Mai J, Mallilankaraman K, Gandhirajan RK, Eguchi S et al (2015) Hyperhomocysteinemia and hyperglycemia induce and potentiate endothelial dysfunction via μ-calpain activation. Diabetes 64:947–959
Tlili A, Jacobs F, de Koning L, Mohamed S, Bui LC, Dairou J, Belin N, Ducros V et al (2013) Hepatocyte-specific Dyrk1a gene transfer rescues plasma apolipoprotein A-I levels and aortic Akt/GSK3 pathways in hyperhomocysteinemic mice. Biochim Biophys Acta 1832:718–728
Acknowledgements
We acknowledge the platform accommodation and animal testing of the animal house at the Institute Jacques-Monod (University Paris Diderot) and the FlexStation3 and PIC2 Facilities of the Functional and Adaptative Biology (BFA) laboratory.
Funding
This work was supported by the Fondation Jerome Lejeune (grant number 2017a-1634).
Author information
Authors and Affiliations
Contributions
AL, YG, and NJ designed the study; AL, YG, NK, FD, CC, and BG performed experiments; SM, VH, JCR, and SM performed yeast two-hybrid analysis; JLP performed HPLC analysis; NJ, AL, YG, JLP, JMD, EY, MA, and MM analyzed the data; NJ and AL wrote the paper; NJ and MM correct the manuscript.
Corresponding author
Ethics declarations
All procedures were carried out in accordance with the ethical standards of French and European regulations (European Communities Council Directive, 86/609/EEC). Official authorization from the French Ministry of Agriculture was granted to perform research and experiments on animals (authorization number 75–369), and the experimental protocol was approved by the institutional animal care and use committee of the Paris Diderot University (CEEA40).
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Table S1
(DOCX 45 kb)
Rights and permissions
About this article
Cite this article
Latour, A., Gu, Y., Kassis, N. et al. LPS-Induced Inflammation Abolishes the Effect of DYRK1A on IkB Stability in the Brain of Mice. Mol Neurobiol 56, 963–975 (2019). https://doi.org/10.1007/s12035-018-1113-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1113-x