Abstract
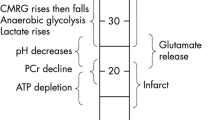
Prolongation of the T2 relaxation time, an increase in T2-weighted signal intensity (T2-SI), and a decrease in the apparent diffusion coefficient (ADC) calculated from diffusion-weighted images (DWI) on magnetic resonance imaging (MRI) are conventional indicators of the vasogenic (interstitial) or cytotoxic (cellular) cerebral edema that develops after ischemic stroke. However, these parameters obtained on stroke imaging have not given us a precise threshold at which we can determine the viability or vulnerability of the tissue, allowing us to decide on an intervention that will help reversible tissue in the acute phase. Here, we introduce a new indicator—the essential diffusion coefficient or EDC, calculated from the T2-SI and ADC—that permits detection of irreversible brain damage after induction of experimental, focal cerebral ischemia. Our three-vessel occlusion (3-VO) method (Yang et al. Eur Neurol 71:4–18, 2014) was applied to investigate early changes on 7-T MRI. In the 3-VO model, which targets only a part of the cortex, animals seldom die at least within 24 h. The T2-SI and the ADC value were monitored, starting at 60 min after reperfusion, and every 30–60 min, for 10 h after the induction of focal ischemia. The region of interest (ROI) was set in each of the following: (1) the ischemic core (the dead zone); (2) the medial border area (the dying/dead mixed zone, including the ischemic penumbra); (3) the lateral border area (the surviving zone after the ischemic stress, where the rCBF is above the threshold for death); and (4) The intact area (outside the ischemic zone). The diagnosis was made by histological analysis performed 24 h after reperfusion. Significant increases in the T2-SI were observed, in ROI-1 at 1 h, in ROI-2 at 2.5 h, and in ROI-3 at 4 h post-reperfusion (1.10, 1.11, or 1.11; > 1.10, respectively, p < 0.001). Significant reductions in the ADC were also observed in ROI-1, ROI-2, and ROI-3, at 1 h post-reperfusion (0.55, 0.52, or 0.58; < 0.60, respectively, p < 0.001), indicating that both types of cerebral edema develop simultaneously in the acute phase. In the EDC analysis, from 5.0 h post-reperfusion, the value in the dying/dead zone (ROI-1 and ROI-2) was consistently reduced to < 50%, showing repeated, significant differences from the value in the surviving zone (ROI-3). A reduction in the EDC to below 50% indicated irreversible tissue damage, with transformation to cerebral infarction. We could detect a sign of cerebral infarction (initial necrosis-like irreversible lesion) as early as 5.25 h after the onset of ischemia. Although the biological time that depends on the body weight must be different between mice and humans, the earliest irreversible tissue damage or tissue destruction (to have achieved the risk of hemorrhagic transformation) that progressed after invisible or silent cell death in the ultra-acute phase, seems to occur at a similar time point.





Similar content being viewed by others
References
Astrup J, Siesjo BK, Symon L (1981) Thresholds in cerebral ischemia—the ischemic penumbra. Stroke 12(6):723–725
Astrip J, Symon L, Branston NM, Lassen NA (1977) Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke 8(1):51–57
von Kummer R, Dzialowski I (2017) Imaging of cerebral ischemic edema and neuronal death. Neuroradiology 59(6):545–553
Betz AL (1996) Alterations in cerebral endothelial cell function in ischemia. Adv Neurol 71:301–311
Quast MJ, Huang NC, Hillman GR, Kent TA (1993) The evolution of acute stroke recorded by multimodal magnetic resonance imaging. Magn Reson Imaging 11(4):465–471
Gill R, Sibson NR, Hatfield RH, Burdett NG, Carpenter TA, Hall LD, Pickard JD (1995) A comparison of the early development of ischaemic damage following permanent middle cerebral artery occlusion in rats as assessed using magnetic resonance imaging and histology. J Cereb Blood Flow Metab 15(1):1–11
Lansberg MG, Albers GW, Beaulieu C, Marks MP (2000) Comparison of diffusion-weighted MRI and CT in acute stroke. Neurology 54(8):1557–1561
Lansberg MG, Thijs VN, O’Brien MW, Ali JO, de Crespigny AJ, Tong DC, Moseley ME, Albers GW (2001) Evolution of apparent diffusion coefficient, diffusion-weighted, and T2-weighted signal intensity of acute stroke. AJNR Am J Neuroradiol 22(4):637–644
Lutsep HL, Albers GW, DeCrespigny A, Kamet GN, Marks MP, Moseley ME (1997) Clinical utility of diffusion-weighted magnetic resonance imaging in the assessment of ischemic stroke. Ann Neurol 41(5):574–580
Kato H, Kogure K, Ohtomo H, Izumiyama M, Tobita M, Matsui S, Yamamoto E, Kohno H, Ikebe Y, Watanabe T (1986) Characterization of experimental ischemic brain edema utilizing proton nuclear magnetic resonance imaging. J Cereb Blood Flow Metab 6(2):212–221
Naruse S, Aoki Y, Takei R, Horikawa Y, Uede S (1991) Effects of atrial natriuretic peptide on ischemic brain edema in rats evaluated by proton magnetic resonance method. Stroke 22(1):61–65
Moseley ME, Kucharczyk J, Mintorovitch J, Cohen Y, Kurhanewicz J, Derugin N, Asgari H, Norman D (1990) Diffusion-weighted MR imaging of acute stroke: correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNR Am J Neuroradiol 11(3):423–429
Roberts TP, Vexler Z, Derugin N, Moseley ME, Kucharczyk J (1993) High-speed MR imaging of ischemic brain injury following stenosis of the middle cerebral artery. J Cereb Blood Flow Metab 13(6):940–946
Lovblad KO, Laubach HJ, Baird AE, Crutin F, Schlaug G, Edelman RR, Warach S (1998) Clinical experience with diffusion-weighted MR in patients with acute stroke. AJNR Am J Neuroradiol 19(6):1061–1066
van Everdingen KJ, van Der Grond J, Kappelle LJ, Ramos LM, Mali WP (1998) Diffusion-weighted magnetic resonance imaging in acute stroke. Stroke 29(9):1783–1790
Tong DC, Yenari MA, Albers GW, O’Brien MW, Marks MP, Moseley ME (1998) Correlation of perfusion- and diffusion-weighted MRI with NIHSS score in acute (< 6.5 hour) ischemic stroke. Neurology 50(4):864–870
Hossmann KA, Fischer M, Bockhorst K, Hoehn-Berlage M (1994) NMR imaging of the apparent diffusion coefficient (ADC) for the evaluation of metabolic suppression and recovery after prolonged cerebral ischemia. J Cereb Blood Flow Metab 14(5):723–731
Busza AL, Allen KL, King MD, van Bruggen N, Williams SR, Gadian DG (1992) Diffusion-weighted imaging studies of cerebral ischemia in gerbils. Potential relevance to energy failure. Stroke 23(11):1602–1612
Mintorovitch J, Yang GY, Shimizu H, Kucharczyk J, Chan PH, Weinstein PR (1994) Diffusion-weighted magnetic resonance imaging of acute focal cerebral ischemia: comparison of signal intensity with changes in brain water and Na+, K(+)-ATPase activity. J Cereb Blood Flow Metab 14(2):332–336
Matsuoka Y, Hossmann KA (1982) Cortical impedance and extracellular volume changes following middle cerebral artery occlusion in cats. J Cereb Blood Flow Metab 2(4):466–474
Sykova E, Svoboda J, Polak J, Chvatal A (1994) Extracellular volume fraction and diffusion characteristics during progressive ischemia and terminal anoxia in the spinal cord of the rat. J Cereb Blood Flow Metab 14(2):301–311
Perez-Trepichio AD, Xue M, Ng TC, Majors AW, Furlan AJ, Awad IA, Jones SC (1995) Sensitivity of magnetic resonance diffusion-weighted imaging and regional relationship between the apparent diffusion coefficient and cerebral blood flow in rat focal cerebral ischemia. Stroke 26(4):667–674
Schlaug G, Siewert B, Benfield A, Edelman RR, Warach S (1997) Time course of the apparent diffusion coefficient (ADC) abnormality in human stroke. Neurology 49(1):113–119
Benveniste H, Hedlund LW, Johnson GA (1992) Mechanism of detection of acute cerebral ischemia in rats by diffusion-weighted magnetic resonance microscopy. Stroke 23(5):746–754
Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M (1986) MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161(2):401–407
Yang D, Nakajo Y, Iihara K, Kataoka H, Nakagawara J, Zhao Q, Yanamoto H (2014) An integrated stroke model with a consistent penumbra for the assessment of neuroprotective interventions. Eur Neurol 71:4–18
Yanamoto H, Nagata I, Niitsu Y, Xue JH, Zhang Z, Kikuchi H (2003) Evaluation of MCAO stroke models in normotensive rats: standardized neocortical infarction by the 3VO technique. Exp Neurol 182(2):261–274
Yanamoto H, Nagata I, Hashimoto N, Kikuchi H (1998) Three-vessel occlusion using a micro-clip for the proximal left middle cerebral artery produces a reliable neocortical infarct in rats. Brain Res Brain Res Protoc 3(2):209–220
Back T (1998) Pathophysiology of the ischemic penumbra—revision of a concept. Cell Mol Neurobiol 18(6):621–638
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20(1):84–91
Koizumi J, Yoshida Y, Nakazawa T, Ooneda G (1986) Experimental studies of ischemic brain edema: a new experimental studies of ischemic brain edema: 1. A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke 8:1–8
Kim JH, Na DG, Chang KH, Song IC, Choi SH, Son KR, Kim KW, Sohn CH (2013) Serial MR analysis of early permanent and transient ischemia in rats: diffusion tensor imaging and high b value diffusion weighted imaging. Korean J Radiol 14(2):307–315
Shibata M, Kumar SR, Amar A, Fernandez JA, Hofman F, Griffin JH, Zlokovic BV (2001) Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation 103(13):1799–1805
Kitagawa K, Matsumoto M, Yang G, Mabuchi T, Yagita Y, Hori M, Yanagihara T (1998) Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: evaluation of the patency of the posterior communicating artery. J Cereb Blood Flow Metab 18(5):570–579
Lin X, Miao P, Wang J, Yuan F, Guan Y, Tang Y, He X, Wang Y, Yang GY (2013) Surgery-related thrombosis critically affects the brain infarct volume in mice following transient middle cerebral artery occlusion. PLoS One 8(9):e75561
Yamato K, Nakajo Y, Yamamoto-Imoto H, Kokame K, Miyata T, Kataoka H, Takahashi JC, Yanamoto Y (2017) A clinically relevant dose of activated protein C (APC) suppresses the development of experimental cerebral infarction. Soc Neurosci 2017
Yanamoto H, Miyamoto S, Nakajo Y, Nakano Y, Hori T, Naritomi H, Kikuchi H (2008) Repeated application of an electric field increases BDNF in the brain, enhances spatial learning, and induces infarct tolerance. Brain Res 1212:79–88
Yang D, Nakajo Y, Iihara K, Kataoka H, Yanamoto H (2013) Alogliptin, a dipeptidylpeptidase-4 inhibitor, for patients with diabetes mellitus type 2, induces tolerance to focal cerebral ischemia in non-diabetic, normal mice. Brain Res 1517:104–113
Nakajo Y, Yang D, Takahashi JC, Zhao Q, Kataoka H, Yanamoto H (2015) ERV enhances spatial learning and prevents the development of infarcts, accompanied by upregulated BDNF in the cortex. Brain Res 1610:110–123
Yamamoto H, Kokame K, Okuda T, Nakajo Y, Yanamoto H, Miyata T (2011) NDRG4 protein-deficient mice exhibit spatial learning deficits and vulnerabilities to cerebral ischemia. J Biol Chem 286(29):26158–26165
Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM (1986) Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 17(6):1304–1308
Hoehn M, Nicolay K, Franke C, van der Sanden B (2001) Application of magnetic resonance to animal models of cerebral ischemia. J Magn Reson Imaging 14(5):491–509
Minematsu K, Li L, Fisher M, Sotak CH, Davis MA, Fiandaca MS (1992) Diffusion-weighted magnetic resonance imaging: rapid and quantitative detection of focal brain ischemia. Neurology 42(1):235–240
Chen F, Suzuki Y, Nagai N, Jin L, Yu J, Wang H, Marchal G, Ni Y (2007) Rodent stroke induced by photochemical occlusion of proximal middle cerebral artery: Evolution monitored with MR imaging and histopathology. Eur J Radiol 63(1):68–75
Pham M, Helluy X, Kleinschnitz C, Kraft P, Bartsch AJ, Jakob P, Nieswandt B, Bendszus M, Stoll G (2011) Sustained reperfusion after blockade of glycoprotein-receptor-Ib in focal cerebral ischemia: an MRI study at 17.6 Tesla. PLoS ONE 6(4):e18386
Pham M, Helluy X, Braeuninger S, Jakob P, Stoll G, Kleinschnitz C, Bendszus M (2010) Outcome of experimental stroke in C57Bl/6 and Sv/129 mice assessed by multimodal ultra-high field MRI. Exp Transl Stroke Med 15:2–6
Yanamoto H, Nagata I, Niitsu Y, Zhang Z, Xue JH, Sakai N, Kikuchi H (2001) Prolonged mild hypothermia therapy protects the brain against permanent focal ischemia. Stroke 32(1):232–239
Griffin JH, Femandez JA, Liu D, Cheng T, Guo H, Zlokovic BV (2004) Activated protein C and ischemic stroke. Crit Care Med 32(5 Suppl):S247–S253
Hoehn-Berlage M, Eis M, Back T, Kohno K, Yamashita K (1995) Changes of relaxation times (T1, T2) and apparent diffusion coefficient after permanent middle cerebral artery occlusion in the rat: temporal evolution, regional extent, and comparison with histology. Magn Reson Med 34(6):824–834
Sakoh M, Ohnishi T, Ostergaard L, Gjedde A (2003) Prediction of tissue survival after stroke based on changes in the apparent diffusion of water (cytotoxic edema). Acta Neurochir Suppl 86:137–140
Gerriets T, Stolz E, Walberer M, Müller C, Kluge A, Kaps M, Fisher M, Bachmann G (2004) Middle cerebral artery occlusion during MR-imaging: investigation of the hyperacute phase of stroke using a new in-bore occlusion model in rats. Brain Res Brain Res Protoc 12(3):137–143
Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D, ECASS Investigators (2008) Thrombolysis with Alteplase 3 to 4.5 hours after acute Ischemic stroke. N Engl J Med 359(13):1317–1329
Calder WA III (1983) Body size, mortality, and longevity. J Theor Biol 102(1):135–144
Calder WA III , (1981) Scaling of physiological processes in homeothermic animals. Annu Rev Physiol 43:301–322
Yanamoto H, Hashimoto N, Kassell NF, Lee KS (1996) Disruption of blood brain barrier triggered by reperfusion following transient focal ischemia in rats. Soc Neurosci 22(Part 3):2143
Dreier JP (2011) The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med 17(4):439–447
Yuzawa I, Sakadzic S, Srinivasan VJ, Shin HK, Eikermann-Haerter K, Boas DA, Ayata C (2012) Cortical spreading depression impairs oxygen delivery and metabolism in mice. J Cereb Blood Flow Metab 32(2):376–386
Hossmann KA (1996) Periinfarct depolarizations. Cerebrovasc Brain Metab Rev 8(3):195–208
Nedergaard M, Hansen AJ (1993) Characterization of cortical depolarizations evoked in focal cerebral ischemia. J Cereb Blood Flow Metab 13(4):568–574
Nedergaard M, Hansen AJ (1988) Spreading depression is not associated with neuronal injury in the normal brain. Brain Res 449(1–2):395–398
Dreier JP, Reiffurth C (2017) Exploitation of the spreading depolarization-induced cytotoxic edema for high-resolution, 3D mapping of its heterogeneous propagation paths. PNAS 114(9):2112–2114
Koroleva VI, Vykhodtseva NI, Elagin VA (1986) Spreading depression in the cortex and subcortical structures of the brain of the rat induced by exposure to focused ultrasound. Neirofiziologiia 18(1):55–61
Vinogradova LV, Koroleva VI, Bures J (1991) Re-entry waves of Leao’s spreading depression between neocortex and caudate nucleus. Brain Res 538(1):161–164
Schielke GP, Moises HC, Betz AL (1991) Blood to brain sodium transport and interstitial fluid potassium concentration during early focal ischemia in the rat. J Cereb Blood Flow Metab 11(3):466–471
Haas M, Forbush BIII (1998) The Na-K-Cl cotransporters. J Bioenerg Biomembr 30(2):161–172
O’Donnell ME, Tran L, Lam TI, Liu XB, Anderson SE (2004) Bumetanide inhibition of the blood-brain barrier Na-K-Cl cotransporter reduces edema formation in the rat middle cerebral artery occlusion model of stroke. J Cereb Blood Flow Metab 24(9):1046–1056
Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L, Tsymbalyuk N, West GA, Gerzanich V (2006) Newly expressed SUR1-regulated NC (Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med 12(4):433–440
Vella J, Zammit C, Di Giovanni G, Muscat R, Valentino M (2015) The central role of aquaporins in the pathophysiology of ischemic stroke. Front Cell Neurosci 9:108
Papadopoulos MC, Verkman AS (2013) Aquaporin water channels in the nervous system. Nat Rev Neurosci 14(4):265–277
Yanamoto H, Miyamoto S, Tohnai N, Nagata I, Xue JH, Nakano Y, Nakajo Y, Kikuchi H (2005) Induced spreading depression activates persistent neurogenesis in the subventricular zone, generating cells with markers for divided and early committed neurons in the caudate putamen and cortex. Stroke 36(7):1544–1550
Leao AAP (1944) Spreading depression of activity in the cerebral cortex. J Neurophysiol 7:359–390
Leao AAP, Morrison RS (1945) Propagation of spreading cortical depression. J Neurophysiol 8:33–45
Dreier JP, Fabricius M, Ayata C, Sakowitz OW, William Shuttleworth C, Dohmen C, Graf R, Vajkoczy P et al (2017) Recording, analysis, and interpretation of spreading depolarizations in neurointensive care: review and recommendations of the COSBID research group. J Cereb Blood Flow Metab 37(5):1595–1625
Memezawa H, Minamisawa H, Smith ML, Siesjo BK (1992) Ischemic penumbra in a model of reversible middle cerebral artery occlusion in the rat. Exp Brain Res 89(1):67–78
Acknowledgements
We thank the valuable assistance of Nozomi Momosaki from the Laboratory of Neurology and Neurosurgery, National Cerebral and Cardiovascular Center. This work was supported by Japan Cardiovascular Research Foundation to HY (#J003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakajo, Y., Zhao, Q., Enmi, Ji. et al. Early Detection of Cerebral Infarction After Focal Ischemia Using a New MRI Indicator. Mol Neurobiol 56, 658–670 (2019). https://doi.org/10.1007/s12035-018-1073-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1073-1