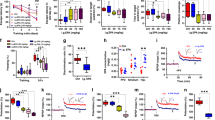
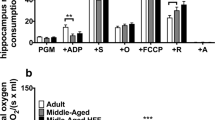
Abstract
Impaired glucose metabolism and mitochondrial decay greatly increase with age, when cognitive decline becomes rampant. No pharmacological or dietary intervention has proven effective, but proper diet and lifestyle do postpone the onset of neurodegeneration and some nutrients are being investigated. We studied insulin signaling, mitochondrial activity and biogenesis, and synaptic signaling in the hippocampus and cortex following dietary supplementation with bioactive phospholipid concentrates of krill oil (KOC), buttermilk fat globule membranes (BMFC), and a combination of both in aged rats. After 3 months of supplementation, although all groups of animals showed clear signs of peripheral insulin resistance, the combination of KOC and BMFC was able to improve peripheral insulin sensitivity. We also explored brain energy balance. Interestingly, the hippocampus of supplemented rats—mainly when supplemented with BMFC or the combination of KOC and BMFC—showed an increase in intracellular adenosine triphosphate (ATP) levels, whereas no difference was observed in the cerebral cortex. Moreover, we found a significant increase of brain-derived neurotrophic factor (BDNF) in the hippocampus of BMFC+KO animals. In summary, dietary supplementation with KOC and/or BMFC improves peripheral and central insulin resistance, suggesting that their administration could delay the onset of these phenomena. Moreover, n-3 fatty acids (FAs) ingested as phospholipids increase BDNF levels favoring an improvement in energy state within neurons and facilitating both mitochondrial and protein synthesis, which are necessary for synaptic plasticity. Thus, dietary supplementation with n-3 FAs could protect local protein synthesis and energy balance within dendrites, favoring neuronal health and delaying cognitive decline associated to age-related disrepair.





Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- Akt:
-
Protein kinase B
- AMPK:
-
AMP-activated protein kinase
- α-Syn:
-
Chaperone α-synuclein
- ATP:
-
Adenosine triphosphate
- BDNF:
-
Brain-derived neurotrophic factor
- BMFC:
-
Buttermilk fat globule concentrate
- CD:
-
Cognitive deficiency
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- FAs:
-
Fatty acids
- Glut4:
-
Glucose transporter type 4
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance index
- IRβ:
-
Insulin receptor-beta subunit
- IRS:
-
Insulin receptor substrate
- KOC:
-
Krill oil concentrate
- MFGM:
-
Milk fat globule membrane
- mTOR:
-
Mammalian target of rapamycin
- PUFA:
-
Polyunsaturated fatty acid
- PC:
-
Phosphatidylcholine
- PS:
-
Phosphatidylserine
- PE:
-
Phosphatidylethanolamine
- PI:
-
Phosphatidylinositol
- PLE:
-
Pressurized liquid extraction
- PI3K:
-
Phosphatidylinositol-3-kinase
- PGC-1α:
-
Proliferator-activated receptor γ coactivator 1-α
- SM:
-
Sphingomyelin
- SIRT1:
-
Sirtuin 1
- Stx1A:
-
Syntaxin 1A
- Syn1:
-
Synapsin I
- Syt1:
-
Synaptotagmin 1
- TAG:
-
Triacylglycerides
- Vamp2:
-
Synaptobrevin 2
References
Bhat AH, Dar KB, Anees S, Zargar MA, Masood A, Sofi MA, Ganie SA (2015) Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed Pharmacother 74:101–110. https://doi.org/10.1016/j.biopha.2015.07.025S0753-3322(15)00169-9
Bratic I, Trifunovic A (2010) Mitochondrial energy metabolism and ageing. Biochim Biophys Acta 1797(6–7):961–967. https://doi.org/10.1016/j.bbabio.2010.01.004S0005-2728(10)00005-8
Ghasemi R, Haeri A, Dargahi L, Mohamed Z, Ahmadiani A (2013) Insulin in the brain: sources, localization and functions. Mol Neurobiol 47(1):145–171. https://doi.org/10.1007/s12035-012-8339-9
Kleinridders A, Ferris HA, Cai W, Kahn CR (2014) Insulin action in brain regulates systemic metabolism and brain function. Diabetes 63(7):2232–2243. https://doi.org/10.2337/db14-0568db14-0568
Akintola AA, van Heemst D (2015) Insulin, aging, and the brain: mechanisms and implications. Front Endocrinol (Lausanne) 6:13. https://doi.org/10.3389/fendo.2015.00013
Calder PC, Bosco N, Bourdet-Sicard R, Capuron L, Delzenne N, Dore J, Franceschi C, Lehtinen MJ, Recker T, Salvioli S, Visioli F (2017) Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res Rev. doi:https://doi.org/10.1016/j.arr.2017.09.001
Gomez LA, Hagen TM (2012) Age-related decline in mitochondrial bioenergetics: does supercomplex destabilization determine lower oxidative capacity and higher superoxide production? Semin Cell Dev Biol 23(7):758–767. https://doi.org/10.1016/j.semcdb.2012.04.002S1084-9521(12)00074-2
Gralle M (2017) The neuronal insulin receptor in its environment. J Neurochem 140(3):359–367. https://doi.org/10.1111/jnc.13909
Unger JW, Livingston JN, Moss AM (1991) Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Prog Neurobiol 36(5):343–362
Pearson-Leary J, McNay EC (2016) Novel roles for the insulin-regulated glucose transporter-4 in hippocampally dependent memory. J Neurosci 36(47):11851–11864. https://doi.org/10.1523/JNEUROSCI.1700-16.2016
Biessels GJ, Reagan LP (2015) Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci 16(11):660–671. https://doi.org/10.1038/nrn4019
Bartke A (2008) Insulin and aging. Cell Cycle 7(21):3338–3343. https://doi.org/10.4161/cc.7.21.7012
Solfrizzi V, Panza F (2014) Mediterranean diet and cognitive decline. A lesson from the whole-diet approach: what challenges lie ahead? J Alzheimers Dis 39(2):283–286. https://doi.org/10.3233/JAD-130831F076J887L8877J0V
Crespo MC, Tome-Carneiro J, Pintado C, Davalos A, Visioli F, Burgos-Ramos E (2017) Hydroxytyrosol restores proper insulin signaling in an astrocytic model of Alzheimer's disease. Biofactors 43(4):540–548. https://doi.org/10.1002/biof.1356
Jicha GA, Markesbery WR (2010) Omega-3 fatty acids: potential role in the management of early Alzheimer’s disease. Clin Interv Aging 5:45–61
Vauzour D, Camprubi-Robles M, Miquel-Kergoat S, Andres-Lacueva C, Banati D, Barberger-Gateau P, Bowman GL, Caberlotto L et al (2017) Nutrition for the ageing brain: Towards evidence for an optimal diet. Ageing Res Rev 35:222–240. https://doi.org/10.1016/j.arr.2016.09.010
FAO, WFP, IFAD (2012) The state of food insecurity in the world 2012. Economic growth is necessary but not sufficient to accelerate reduction of hunger and malnutrition. http://www.faoorg/docrep/016/i3027e/i3027epdf
Riediger ND, Othman RA, Suh M, Moghadasian MH (2009) A systemic review of the roles of n-3 fatty acids in health and disease. J Am Diet Assoc 109(4):668–679. https://doi.org/10.1016/j.jada.2008.12.022S0002-8223(08)02335-3
Wijendran V, Huang MC, Diau GY, Boehm G, Nathanielsz PW, Brenna JT (2002) Efficacy of dietary arachidonic acid provided as triglyceride or phospholipid as substrates for brain arachidonic acid accretion in baboon neonates. Pediatr Res 51(3):265–272. https://doi.org/10.1203/00006450-200203000-00002
Berge K, Musa-Veloso K, Harwood M, Hoem N, Burri L (2014) Krill oil supplementation lowers serum triglycerides without increasing low-density lipoprotein cholesterol in adults with borderline high or high triglyceride levels. Nutr Res 34(2):126–133. https://doi.org/10.1016/j.nutres.2013.12.003
Deutsch L (2007) Evaluation of the effect of Neptune Krill Oil on chronic inflammation and arthritic symptoms. J Am Coll Nutr 26(1):39–48
Konagai C, Yanagimoto K, Hayamizu K, Han L, Tsuji T, Koga Y (2013) Effects of krill oil containing n-3 polyunsaturated fatty acids in phospholipid form on human brain function: a randomized controlled trial in healthy elderly volunteers. Clin Interv Aging 8:1247–1257. https://doi.org/10.2147/CIA.S50349cia-8-1247
Castro-Gomez P, Garcia-Serrano A, Visioli F, Fontecha J (2015) Relevance of dietary glycerophospholipids and sphingolipids to human health. Prostaglandins Leukot Essent Fatty Acids 101:41–51. doi:https://doi.org/10.1016/j.plefa.2015.07.004S0952-3278(15)00138-6
Castro-Gomez P, Rodriguez-Alcala LM, Monteiro KM, Ruiz AL, Carvalho JE, Fontecha J (2016) Antiproliferative activity of buttermilk lipid fractions isolated using food grade and non-food grade solvents on human cancer cell lines. Food Chem 212:695–702. https://doi.org/10.1016/j.foodchem.2016.06.030S0308-8146(16)30922-0
Osella MC, Re G, Badino P, Bergamasco L, Miolo A (2008) Phosphatidylserine (PS) as a potential nutraceutical for canine brain aging: a review. J Vet Behav: Clin Appl 3(2):41–51. https://doi.org/10.1016/j.jveb.2007.08.003
Lu PH, Lee GJ, Raven EP, Tingus K, Khoo T, Thompson PM, Bartzokis G (2011) Age-related slowing in cognitive processing speed is associated with myelin integrity in a very healthy elderly sample. J Clin Exp Neuropsychol 33(10):1059–1068. https://doi.org/10.1080/13803395.2011.595397
Castro-Gomez MP, Rodriguez-Alcala LM, Calvo MV, Romero J, Mendiola JA, Ibanez E, Fontecha J (2014) Total milk fat extraction and quantification of polar and neutral lipids of cow, goat, and ewe milk by using a pressurized liquid system and chromatographic techniques. J Dairy Sci 97(11):6719–6728. https://doi.org/10.3168/jds.2014-8128S0022-0302(14)00600-6
Umegaki H, Makino T, Uemura K, Shimada H, Hayashi T, Cheng XW, Kuzuya M (2017) The associations among insulin resistance, hyperglycemia, physical performance, diabetes mellitus, and cognitive function in relatively healthy older adults with subtle cognitive dysfunction. Front Aging Neurosci 9:72. https://doi.org/10.3389/fnagi.2017.00072
Burgos-Ramos E, Chowen JA, Arilla-Ferreiro E, Canelles S, Argente J, Barrios V (2011) Chronic central leptin infusion modifies the response to acute central insulin injection by reducing the interaction of the insulin receptor with IRS2 and increasing its association with SOCS3. J Neurochem 117(1):175–185. https://doi.org/10.1111/j.1471-4159.2011.07191.x
Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P et al (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458(7241):1056–1060. https://doi.org/10.1038/nature07813
Marosi K, Mattson MP (2014) BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab 25(2):89–98. https://doi.org/10.1016/j.tem.2013.10.006S1043-2760(13)00178-1
Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H (2004) Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci 24(44):9760–9769. https://doi.org/10.1523/JNEUROSCI.1427-04.2004
Fontana L, Kennedy BK, Longo VD, Seals D, Melov S (2014) Medical research: treat ageing. Nature 511(7510):405–407. https://doi.org/10.1038/511405a
Daviglus ML, Plassman BL, Pirzada A, Bell CC, Bowen PE, Burke JR, Connolly ES Jr, Dunbar-Jacob JM et al (2011) Risk factors and preventive interventions for Alzheimer disease: state of the science. Arch Neurol 68(9):1185–1190. https://doi.org/10.1001/archneurol.2011.100
Jackson PA, Pialoux V, Corbett D, Drogos L, Erickson KI, Eskes GA, Poulin MJ (2016) Promoting brain health through exercise and diet in older adults: a physiological perspective. J Physiol 594(16):4485–4498. https://doi.org/10.1113/JP271270
Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V et al (2013) Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 368(14):1279–1290. https://doi.org/10.1056/NEJMoa1200303
Schubert M, Contreras C, Franz N, Hellhammer J (2011) Milk-based phospholipids increase morning cortisol availability and improve memory in chronically stressed men. Nutr Res 31(6):413–420. https://doi.org/10.1016/j.nutres.2011.05.012S0271-5317(11)00115-1
Hernell O, Timby N, Domellof M, Lonnerdal B (2016) Clinical benefits of milk fat globule membranes for infants and children. J Pediatr 173(Suppl):S60–S65. https://doi.org/10.1016/j.jpeds.2016.02.077S0022-3476(16)00300-0
Kim H, Suzuki T, Kim M, Kojima N, Ota N, Shimotoyodome A, Hase T, Hosoi E et al (2015) Effects of exercise and milk fat globule membrane (MFGM) supplementation on body composition, physical function, and hematological parameters in community-dwelling frail Japanese women: a randomized double blind, placebo-controlled, follow-up trial. PLoS One 10(2):e0116256. https://doi.org/10.1371/journal.pone.0116256PONE-D-14-31959
Minegishi Y, Ota N, Soga S, Shimotoyodome A (2016) Effects of nutritional supplementation with milk fat globule membrane on physical and muscle function in healthy adults aged 60 and over with semiweekly light exercise: a randomized double-blind, placebo-controlled pilot trial. J Nutr Sci Vitaminol (Tokyo) 62(6):409–415. https://doi.org/10.3177/jnsv.62.409
Lobraico JM, DiLello LC, Butler AD, Cordisco ME, Petrini JR, Ahmadi R (2015) Effects of krill oil on endothelial function and other cardiovascular risk factors in participants with type 2 diabetes, a randomized controlled trial. BMJ Open Diabetes Res Care 3(1):e000107. https://doi.org/10.1136/bmjdrc-2015-000107bmjdrc-2015-000107
Demmer E, Van Loan MD, Rivera N, Rogers TS, Gertz ER, German JB, Smilowitz JT, Zivkovic AM (2016) Addition of a dairy fraction rich in milk fat globule membrane to a high-saturated fat meal reduces the postprandial insulinaemic and inflammatory response in overweight and obese adults. J Nutr Sci 5:e14. https://doi.org/10.1017/jns.2015.4200042
Lamaziere A, Richard D, Barbe U, Kefi K, Bausero P, Wolf C, Visioli F (2011) Differential distribution of DHA-phospholipids in rat brain after feeding: a lipidomic approach. Prostaglandins Leukot Essent Fatty Acids 84 (1–2):7–11. doi:https://doi.org/10.1016/j.plefa.2010.11.001
Orlando M, Lignani G, Maragliano L, Fassio A, Onofri F, Baldelli P, Giovedi S, Benfenati F (2014) Functional role of ATP binding to synapsin I in synaptic vesicle trafficking and release dynamics. J Neurosci 34(44):14752–14768. https://doi.org/10.1523/JNEUROSCI.1093-14.201434/44/14752
Wu A, Ying Z, Gomez-Pinilla F (2004) Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma 21(10):1457–1467. https://doi.org/10.1089/neu.2004.21.1457
Acknowledgements
Buttermilk powder was a kind gift of Reny Picot (Oviedo, Spain), and Antarctic krill oil from Euphausia superba was kindly donated by AKO3™ (Aker Biomarine Antarctic AS, Oslo, Norway).
Funding
This study was supported in part by grants from the Spanish Ministerio de Economía y Competitividad (AGL2014-56464-R1 and AGL2014-54565-R2); by the Comunidad de Madrid through the “Programa de actividades en tecnologias” (ALIBIRD-CM S2013/ABI-2728); and by the Castilla-La Mancha University through the “Programa de ayuda I+D a grupos emergentes” (GI20173998) to EBR. AD lab is supported in part by the Spanish Agencia Estatal de Investigación and European Feder Funds (AGL2016-78922-R) and Fundación Ramón Areces (Madrid, Spain).
Author information
Authors and Affiliations
Contributions
JF, CV, and FV designed the study. JTC, MCC, EBR, IPP, SB AGS, PCG, and AV performed experiments. MCC, JTC, EBR, CTZ, AD, and FV wrote the manuscript. All authors approved the submission of the final version of the manuscript.
Corresponding author
Ethics declarations
Animal experiments were approved by the Animal Experimentation Committee of the National Distance Education University (UNED, Spain).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Joao Tomé-Carneiro and M. Carmen Crespo contribute equally to this work.
Electronic Supplementary Material
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Tomé-Carneiro, J., Carmen Crespo, M., Burgos-Ramos, E. et al. Buttermilk and Krill Oil Phospholipids Improve Hippocampal Insulin Resistance and Synaptic Signaling in Aged Rats. Mol Neurobiol 55, 7285–7296 (2018). https://doi.org/10.1007/s12035-018-0934-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-0934-y