Abstract
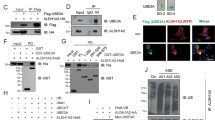
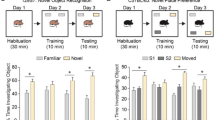
Angelman syndrome (AS) is a complex genetic disorder that affects the nervous system. AS affects an estimated 1 in 12,000 to 20,000 individuals. Characteristic features of AS includes developmental delay or intellectual disability, severe speech impairment, seizures, small head size (microcephaly), and problems with movement and balance (ataxia). AS individuals usually have microdeletion of the maternal copy of 15q11.2–15q13 region of chromosome 15. The E6-associated protein (E6AP, an E3 ubiquitin protein ligase enzyme) is encoded by the gene UBE3A, which is located in this region, and it has been shown that deregulation of E6AP gives rise to AS and neuropathology of autism spectrum disorders (ASDs) (e.g., autism and Rett syndromes). We have shown that E6AP also acts as a coactivator of the estrogen receptor (ER). ER is a ligand-induced transcription factor that exerts potent and wide-ranging effects on the developing brain. Furthermore, the expression pattern of ER in the brain overlaps with that of E6AP. Up till now, all the published studies have examined the role of the ubiquitin-protein ligase activity of E6AP in the development of AS, and it is not known what role the newly discovered coactivation functions of E6AP and ER plays in the pathology of AS. Here, we demonstrate that E6AP and ER co-immunoprecipitate and are in the same protein complex in neuronal cells (Neuro2a). In addition, both colocalize in nuclear and cytoplasmic compartments of the mouse hippocampal neurons and Neuro2a cells. Moreover, we identified a novel E6AP and ER direct transcriptional regulation of a gene Cyp26b1 known to be involved in learning and memory processes. This transcriptional regulation involves recruitment of E6AP and ER to a newly discovered functional estrogen response element (ERE) located at the Cyp26b1 gene promoter and is associated with transcription permissive epigenetic events leading to increase of active transcription of the gene in neurons upon estrogen treatment. This novel transcriptional regulation was also validated in the AS mouse model where E6AP expression is abrogated in the mouse brain. In fact, Cyp26b1 expression is decreased by 31% in AS mice versus age-matched control (Ctrl) mice hippocampi. Also, retinoic acid transcriptional signaling was shown to be amplified as evidenced by specific increased Rarβ and decreased Erbb4 mRNA expression in AS mice versus Ctrl mice hippocampi. These transcript level changes were also supported by the same trend of changes at the protein level. Collectively, our data present a proof of principle that the transcriptional coactivation function of E6AP may have a crucial role in the pathobiology of AS. This function, yet to be thoroughly investigated, reveals the possibility of harnessing the antagonistic estrogen-retinoic acid transcriptional signaling crosstalk and potentially other unknown effectors for the investigation of important possible targets as putative novel treatment modalities and venues for reversing neurological manifestations in AS and related syndromes like ASDs.






Similar content being viewed by others
References
Clayton-Smith J, Laan L (2003) Angelman syndrome: a review of the clinical and genetic aspects. J Med Genet 40(2):87–95. https://doi.org/10.1136/jmg.40.2.87
Williams CA (2005) Neurological aspects of the Angelman syndrome. Brain and Development 27(2):88–94. https://doi.org/10.1016/j.braindev.2003.09.014
Ohta, T., Buiting K., Kokkonen H., McCandless S., Heeger S., Leisti H., Driscoll D.J., Cassidy S.B., et al. Molecular mechanism of angelman syndrome in two large families involves an imprinting mutation. Am J Hum Genet, 1999. 64(2): p. 385–396, DOI: https://doi.org/10.1086/302232.
Sutcliffe, J.S., Jiang Y.h., Galjaard R.J., Matsuura T., Fang P., Kubota T., Christian S.L., Bressler J., et al. The E6-Ap ubiquitin-protein ligase (UBE3A) gene is localized within a narrowed Angelman syndrome critical region. Genome Res, 1997. 7(4): p. 368–377, DOI: https://doi.org/10.1101/gr.7.4.368.
Matsuura T, Sutcliffe JS, Fang P, Galjaard RJ, Jiang Yh, Benton CS, Rommens JM, Beaudet AL (1997) De novo truncating mutations in E6-AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nat Genet 15(1):74–77. https://doi.org/10.1038/ng0197-74
Jiang Y et al (1998) Imprinting in Angelman and Prader-Willi syndromes. Curr Opin Genet Dev 8(3):334–342. https://doi.org/10.1016/S0959-437X(98)80091-9
Lalande M, Calciano MA (2007) Molecular epigenetics of Angelman syndrome. Cell Mol Life Sci 64(7–8):947–960. https://doi.org/10.1007/s00018-007-6460-0
Jiang YH et al (1998) Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron 21(4):799–811. https://doi.org/10.1016/S0896-6273(00)80596-6
McEwen BS, Alves SE (1999) Estrogen actions in the central nervous system. Endocr Rev 20(3):279–307. https://doi.org/10.1210/edrv.20.3.0365
McCarthy MM (2008) Estradiol and the developing brain. Physiol Rev 88(1):91–124. https://doi.org/10.1152/physrev.00010.2007
McCarthy MM, Schwarz JM, Wright CL, Dean SL (2008) Mechanisms mediating oestradiol modulation of the developing brain. J Neuroendocrinol 20(6):777–783. https://doi.org/10.1111/j.1365-2826.2008.01723.x
Mangelsdorf, D.J., Thummel C., Beato M., Herrlich P., Schütz G., Umesono K., Blumberg B., Kastner P., et al., The nuclear receptor superfamily: the second decade. Cell, 1995. 83(6): p. 835–839, DOI: https://doi.org/10.1016/0092-8674(95)90199-X.
Greer PL et al The Angelman syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell 140(5):704–716
Nawaz Z, Lonard DM, Dennis AP, Smith CL, O’Malley BW (1999) Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci U S A 96(5):1858–1862. https://doi.org/10.1073/pnas.96.5.1858
Obeid, J.P., Zeidan Y.H., Zafar N., el Hokayem J., E6-associated protein dependent estrogen receptor regulation of protein kinase a regulatory subunit R2A expression in neuroblastoma. Mol Neurobiol, 2017, DOI: https://doi.org/10.1007/s12035-017-0449-y.
Behl C (2002) Oestrogen as a neuroprotective hormone. Nat Rev Neurosci 3(6):433–442. https://doi.org/10.1038/nrn846
Mardirossian S, Rampon C, Salvert D, Fort P, Sarda N (2009) Impaired hippocampal plasticity and altered neurogenesis in adult Ube3a maternal deficient mouse model for Angelman syndrome. Exp Neurol 220(2):341–348. https://doi.org/10.1016/j.expneurol.2009.08.035
Nawaz Z, Stancel GM, McDonnell DP, Hyder SM (1993) Creation of an active estrogen-responsive element by a single base change in the flanking sequence of a cellular oncogene: a possible mechanism for hormonal carcinogenesis? Mol Carcinog 7(2):76–82. https://doi.org/10.1002/mc.2940070204
Portales-Casamar, E., Thongjuea S., Kwon A.T., Arenillas D., Zhao X., Valen E., Yusuf D., Lenhard B., et al., JASPAR 2010: the greatly expanded open-access database of transcription factor binding profiles. Nucleic Acids Res, 2010. 38(Database issue): p. D105–D110, DOI: https://doi.org/10.1093/nar/gkp950.
Frith MC, Li MC, Weng Z (2003) Cluster-Buster: finding dense clusters of motifs in DNA sequences. Nucleic Acids Res 31(13):3666–3668. https://doi.org/10.1093/nar/gkg540
Dhananjayan SC, Ramamoorthy S, Khan OY, Ismail A, Sun J, Slingerland J, O’Malley BW, Nawaz Z (2006) WW domain binding protein-2, an E6-associated protein interacting protein, acts as a coactivator of estrogen and progesterone receptors. Mol Endocrinol 20(10):2343–2354. https://doi.org/10.1210/me.2005-0533
Rhinn M, Dolle P (2012) Retinoic acid signalling during development. Development 139(5):843–858. https://doi.org/10.1242/dev.065938
Maclean G, Dolle P, Petkovich M (2009) Genetic disruption of CYP26B1 severely affects development of neural crest derived head structures, but does not compromise hindbrain patterning. Dev Dyn 238(3):732–745. https://doi.org/10.1002/dvdy.21878
Shearer KD, Stoney PN, Morgan PJ, McCaffery PJ (2012) A vitamin for the brain. Trends Neurosci 35(12):733–741. https://doi.org/10.1016/j.tins.2012.08.005
Yashiro, K., Zhao X., Uehara M., Yamashita K., Nishijima M., Nishino J., Saijoh Y., Sakai Y., et al., Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing mouse limb. Dev Cell, 2004. 6(3): p. 411–422, DOI: https://doi.org/10.1016/S1534-5807(04)00062-0.
Dagli A, Buiting K, Williams CA (2012) Molecular and clinical aspects of Angelman syndrome. Mol Syndromol 2(3–5):100–112. https://doi.org/000328837
Goodman AB (2005) Microarray results suggest altered transport and lowered synthesis of retinoic acid in schizophrenia. Mol Psychiatry 10(7):620–621. https://doi.org/10.1038/sj.mp.4001668
Offterdinger M et al (1999) Expression of c-erbB-4/HER4 is regulated in T47D breast carcinoma cells by retinoids and vitamin D3. Biochem Biophys Res Commun 258(3):559–564. https://doi.org/10.1016/S0006-291X(00)90001-9
Abu-Abed S, MacLean G, Fraulob V, Chambon P, Petkovich M, Dollé P (2002) Differential expression of the retinoic acid-metabolizing enzymes CYP26A1 and CYP26B1 during murine organogenesis. Mech Dev 110(1–2):173–177. https://doi.org/10.1016/S0925-4773(01)00572-X
MacLean G, Abu-Abed S, Dollé P, Tahayato A, Chambon P, Petkovich M (2001) Cloning of a novel retinoic-acid metabolizing cytochrome P450, Cyp26B1, and comparative expression analysis with Cyp26A1 during early murine development. Mech Dev 107(1–2):195–201. https://doi.org/10.1016/S0925-4773(01)00463-4
Ross AC, Zolfaghari R (2011) Cytochrome P450s in the regulation of cellular retinoic acid metabolism. Annu Rev Nutr 31(1):65–87. https://doi.org/10.1146/annurev-nutr-072610-145127
Goodman T, Crandall JE, Nanescu SE, Quadro L, Shearer K, Ross A, McCaffery P (2012) Patterning of retinoic acid signaling and cell proliferation in the hippocampus. Hippocampus 22(11):2171–2183. https://doi.org/10.1002/hipo.22037
Crandall J, Sakai Y, Zhang J, Koul O, Mineur Y, Crusio WE, McCaffery P (2004) 13-cis-retinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice. Proc Natl Acad Sci U S A 101(14):5111–5116. https://doi.org/10.1073/pnas.0306336101
McCaffery PJ, Adams J, Maden M, Rosa-Molinar E (2003) Too much of a good thing: retinoic acid as an endogenous regulator of neural differentiation and exogenous teratogen. Eur J Neurosci 18(3):457–472. https://doi.org/10.1046/j.1460-9568.2003.02765.x
Olson CR, Mello CV (2010) Significance of vitamin A to brain function, behavior and learning. Mol Nutr Food Res 54(4):489–495. https://doi.org/10.1002/mnfr.200900246
de The H, Marchio A, Tiollais P, Dejean A (1989) Differential expression and ligand regulation of the retinoic acid receptor alpha and beta genes. EMBO J 8(2):429–433
Alfos S, Boucheron C, Pallet V, Higueret D, Enderlin V, Beracochea D, Jaffard R, Higueret P (2001) A retinoic acid receptor antagonist suppresses brain retinoic acid receptor overexpression and reverses a working memory deficit induced by chronic ethanol consumption in mice. Alcohol Clin Exp Res 25(10):1506–1514. https://doi.org/10.1111/j.1530-0277.2001.tb02154.x
Estruch I et al (2017) Characterization of the differential coregulator binding signatures of the retinoic acid receptor subtypes upon (ant)agonist action. Biochim Biophys Acta 1865(9):1195–1206. https://doi.org/10.1016/j.bbapap.2017.06.011
Kaphzan H, Hernandez P, Jung JI, Cowansage KK, Deinhardt K, Chao MV, Abel T, Klann E (2012) Reversal of impaired hippocampal long-term potentiation and contextual fear memory deficits in Angelman syndrome model mice by ErbB inhibitors. Biol Psychiatry 72(3):182–190. https://doi.org/10.1016/j.biopsych.2012.01.021
Emmanouil-Nikoloussi EN et al (2000) Craniofacial abnormalities induced by retinoic acid: a preliminary histological and scanning electron microscopic (SEM) study. Exp Toxicol Pathol 52(5):445–453. https://doi.org/10.1016/S0940-2993(00)80080-9
Foster TC, Rani A, Kumar A, Cui L, Semple-Rowland SL (2008) Viral vector-mediated delivery of estrogen receptor-alpha to the hippocampus improves spatial learning in estrogen receptor-alpha knockout mice. Mol Ther 16(9):1587–1593. https://doi.org/10.1038/mt.2008.140
James, A.W., Theologis A.A., Brugmann S.A., Xu Y., Carre A.L., Leucht P., Hamilton K., Korach K.S., et al., Estrogen/estrogen receptor alpha signaling in mouse posterofrontal cranial suture fusion. PLoS One, 2009. 4(9): p. e7120, DOI: https://doi.org/10.1371/journal.pone.0007120.
Ogawa S, Chan J, Gustafsson JÅ, Korach KS, Pfaff DW (2003) Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology 144(1):230–239. https://doi.org/10.1210/en.2002-220519
Acknowledgements
This work is supported by a postdoctoral fellowship from Foundation for Angelman Syndrome Therapeutics (FAST) granted to Dr. Jimmy El Hokayem and a grant from FAST to Dr. Zafar Nawaz.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(PPTX 5605 kb).
Rights and permissions
About this article
Cite this article
El Hokayem, J., Weeber, E. & Nawaz, Z. Loss of Angelman Syndrome Protein E6AP Disrupts a Novel Antagonistic Estrogen-Retinoic Acid Transcriptional Crosstalk in Neurons. Mol Neurobiol 55, 7187–7200 (2018). https://doi.org/10.1007/s12035-018-0871-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-0871-9