Abstract
The dopamine system has been characterized in motor function, goal-directed behaviors, and rewards. Recent studies recognize various dopamine system genes as being associated with autism spectrum disorder (ASD). However, how dopamine system dysfunction induces ASD pathophysiology remains unknown. In the present study, we demonstrated that mice with increased dopamine functions in the dorsal striatum via the suppression of dopamine transporter expression in substantia nigra neurons or the optogenetic stimulation of the nigro-striatal circuitry exhibited sociability deficits and repetitive behaviors relevant to ASD pathology in animal models, while these behavioral changes were blocked by a D1 receptor antagonist. Pharmacological activation of D1 dopamine receptors in normal mice or the genetic knockout (KO) of D2 dopamine receptors also produced typical autistic-like behaviors. Moreover, the siRNA-mediated inhibition of D2 dopamine receptors in the dorsal striatum was sufficient to replicate autistic-like phenotypes in D2 KO mice. Intervention of D1 dopamine receptor functions or the signaling pathways-related D1 receptors in D2 KO mice produced anti-autistic effects. Together, our results indicate that increased dopamine function in the dorsal striatum promotes autistic-like behaviors and that the dorsal striatum is the neural correlate of ASD core symptoms.





Similar content being viewed by others
References
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric, Washington DC
Zoghbi HY, Bear MF (2012) Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb Perspect Biol 4:a009886. https://doi.org/10.1101/cshperspect.a009886
De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, Kou Y, Liu L et al (2014) Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515:209–215. https://doi.org/10.1038/nature13772
Ebert DH, Greenberg ME (2013) Activity-dependent neuronal signalling and autism spectrum disorder. Nature 493:327–337. https://doi.org/10.1038/nature11860
Kim KC, Gonzales EL, Lázaro MT, Choi CS, Bahn GH, Yoo HJ, Shin CY (2016) Clinical and neurobiological relevance of current animal models of autism spectrum disorders. Biomol Ther 24:207–243. https://doi.org/10.4062/biomolther.2016.061
Wise RA (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5:483–494
Balleine BW, Delgado MR, Hikosaka O (2007) The role of the dorsal striatum in reward and decision-making. J Neurosci 27:8161–8165
Yin HH, Knowlton BJ (2006) The role of the basal ganglia in habit formation. Nat Rev Neurosci 7:464–476
Hettinger JA, Liu X, Schwartz CE, Michaelis RC, Holden JJ (2008) A DRD1 haplotype is associated with risk for autism spectrum disorders in male-only affected sib-pair families. Am J Med Genet B Neuropsychiatr Genet 147B:628–636. https://doi.org/10.1002/ajmg.b.30655
Hettinger JA, Liu X, Hudson ML, Lee A, Cohen IL, Michaelis RC, Schwartz CE, Lewis SM et al (2012) DRD2 and PPP1R1B (DARPP-32) polymorphisms independently confer increased risk for autism spectrum disorders and additively predict affected status in male-only affected sib-pair families. Behav Brain Funct 8:19. https://doi.org/10.1186/1744-9081-8-19
Neale BM, Kou Y, Liu L, Ma'ayan A, Samocha KE, Sabo A, Lin CF, Stevens C et al (2012) Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485:242–245. https://doi.org/10.1038/nature11011
Hamilton PJ, Campbell NG, Sharma S, Erreger K, Herborg Hansen F, Saunders C, Belovich AN, NIH ARRA Autism Sequencing Consortium et al (2013) De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Mol Psychiatry 18:1315–1323. https://doi.org/10.1038/mp.2013.102
Fuccillo MV (2016) Striatal circuits as a common node for autism pathophysiology. Front Neurosci 10:27. https://doi.org/10.3389/fnins.2016.00027
Kim H, Lee Y, Park JY, Kim JE, Kim TK, Choi J, Lee JE, Lee EH et al (2016) Loss of adenylyl cyclase type-5 in the dorsal striatum produces autistic-like behaviors. Mol Neurobiol. https://doi.org/10.1007/s12035-016-0256-x
Sears LL, Vest C, Mohamed S, Bailey J, Ranson BJ, Piven J (1999) An MRI study of the basal ganglia in autism. Prog Neuro-Psychopharmacol Biol Psychiatry 23:613–624
Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, Licalzi E, Wasserman S, Soorya L et al (2005) Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biol Psychiatry 58:226–232
Langen M, Schnack HG, Nederveen H, Bos D, Lahuis BE, de Jonge MV, van Engeland H, Durston S (2009) Changes in the developmental trajectories of striatum in autism. Biol Psychiatry 66:327–333. https://doi.org/10.1016/j.biopsych.2009.03.017
Estes A, Shaw DW, Sparks BF, Friedman S, Giedd JN, Dawson G, Bryan M, Dager SR (2011) Basal ganglia morphometry and repetitive behavior in young children with autism spectrum disorder. Autism Res 4:212–220. https://doi.org/10.1002/aur.193
Langen M, Bos D, Noordermeer SD, Nederveen H, van Engeland H, Durston S (2014) Changes in the development of striatum are involved in repetitive behavior in autism. Biol Psychiatry 76:405–411. https://doi.org/10.1016/j.biopsych.2013.08.013
Schuetze M, Park MT, Cho IY, MacMaster FP, Chakravarty MM, Bray SL (2016) Morphological alterations in the thalamus, striatum, and pallidum in autism spectrum disorder. Neuropsychopharmacology 41:2627–2637. https://doi.org/10.1038/npp.2016.64
Lee KW, Hong JH, Choi IY, Che Y, Lee JK, Yang SD, Song CW, Kang HS et al (2002) Impaired D2 dopamine receptor function in mice lacking type 5 adenylyl cyclase. J Neurosci 22:7931–7940
Kim H, Kim TK, Kim JE, Park JY, Lee Y, Kang M, Kim KS, Han PL (2014) Adenylyl cyclase-5 in the dorsal striatum function as a molecular switch for the generation of behavioral preferences for cue-directed food choices. Mol Brain 7:77. https://doi.org/10.1186/s13041-014-0077-7
Drago J, Gerfen CR, Lachowicz JE, Steiner H, Hollon TR, Love PE, Ooi GT, Grinberg A et al (1994) Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc Natl Acad Sci U S A 91:12564–12568
Kelly MA, Rubinstein M, Asa SL, Zhang G, Saez C, Bunzow JR, Allen RG, Hnasko R et al (1997) Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron 19:103–113
Kim KS, Lee KW, Lee KW, Im JY, Yoo JY, Kim SW, Lee JK, Nestler EJ et al (2006) Adenylyl cyclase type 5 (AC5) is an essential mediator of morphine action. Proc Natl Acad Sci U S A 103:3908–3913
Kim H, Lee Y, Kim JE, Han PL (2016) Reversal of an unconditioned behavioral preference for specific food pellets by intervention of whisker sensory inputs. Exp Neurobiol 25:79–85. https://doi.org/10.5607/en.2016.25.2.79
Ming M, Li X, Fan X, Yang D, Li L, Chen S, Gu Q, Le W (2009) Retinal pigment epithelial cells secrete neurotrophic factors and synthesize dopamine: possible contribution to therapeutic effects of RPE cell transplantation in Parkinson’s disease. J Transl Med 7:53. https://doi.org/10.1186/1479-5876-7-53
Park JY, Kim TK, Choi J, Lee JE, Kim H, Lee EH, Han PL (2014) Implementation of a two-dimensional behavior matrix to distinguish individuals with differential depression states in a rodent model of depression. Exp Neurobiol 23:215–223. https://doi.org/10.5607/en.2014.23.3.215
McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN (2008) Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav 7:152–163
Baek IS, Park JY, Han PL (2015) Chronic antidepressant treatment in normal mice induces anxiety and impairs stress-coping ability. Exp Neurobiol 24:156–168. https://doi.org/10.5607/en.2015.24.2.156
Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG (1998) Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci U S A 95:4029–4034
Kalivas PW, Stewart J (1991) Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev 16:223–244
Grace AA (1991) Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience 41:1–24
Goto Y, Grace AA (2005) Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci 8:805–812
Goto Y, Otani S, Grace AA (2007) The Yin and Yang of dopamine release: a new perspective. Neuropharmacology 53:583–587
Wang H, Wu LJ, Kim SS, Lee FJ, Gong B, Toyoda H, Ren M, Shang YZ et al (2008) FMRP acts as a key messenger for dopamine modulation in the forebrain. Neuron 59:634–647. https://doi.org/10.1016/j.neuron.2008.06.027
Kao FC, Su SH, Carlson GC, Liao W (2015) MeCP2-mediated alterations of striatal features accompany psychomotor deficits in a mouse model of Rett syndrome. Brain Struct Funct 220:419–434. https://doi.org/10.1007/s00429-013-0664-x
Bariselli S, Tzanoulinou S, Glangetas C, Prévost-Solié C, Pucci L, Viguié J, Bezzi P, O'Connor EC et al (2016) SHANK3 controls maturation of social reward circuits in the VTA. Nat Neurosci 19:926–934. https://doi.org/10.1038/nn.4319
Rothwell PE, Fuccillo MV, Maxeiner S, Hayton SJ, Gokce O, Lim BK, Fowler SC, Malenka RC et al (2014) Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell 158:198–212. https://doi.org/10.1016/j.cell.2014.04.045
Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z et al (2011) Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 472:437–442. https://doi.org/10.1038/nature09965
Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R (2009) Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology 204:361–373. https://doi.org/10.1007/s00213-009-1466-y
Rapanelli M, Frick LR, Xu M, Groman SM, Jindachomthong K, Tamamaki N, Tanahira C, Taylor JR et al (2017) Targeted interneuron depletion in the dorsal striatum produces autism-like behavioral abnormalities in male but not female mice. Biol Psychiatry. https://doi.org/10.1016/j.biopsych.2017.01.020
Pavăl D (2017) A dopamine hypothesis of autism spectrum disorder. Dev Neurosci 28. https://doi.org/10.1159/000478725
Jia JM, Zhao J, Hu Z, Lindberg D, Li Z (2013) Age-dependent regulation of synaptic connections by dopamine D2 receptors. Nat Neurosci 16:1627–1636. https://doi.org/10.1038/nn.3542
Sung YK, Kyou CC, Min SC, Myoung HK, Sa YK, Na YS, Jong EL, Byung KJ et al (2006) The dopamine D2 receptor regulates the development of dopaminergic neurons via extracellular signal-regulated kinase and Nurr1 activation. J Neurosci 26:4567–4576
Vengeliene V, Bespalov A, Roßmanith M, Horschitz S, Berger S, Relo AL, Noori HR, Schneider P et al (2017) Towards trans-diagnostic mechanisms in psychiatry: neurobehavioral profile of rats with a loss-of-function point mutation in the dopamine transporter gene. Dis Model Mech 10:451–461. https://doi.org/10.1242/dmm.027623
Dickinson SD, Sabeti J, Larson GA, Giardina K, Rubinstein M, Kelly MA, Grandy DK, Low MJ et al (1999) Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J Neurochem 72:148–156
Acknowledgements
This research was supported by a grant (HI15C1834) from the Ministry of Health and Welfare, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary Figure S1
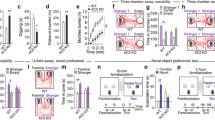
Reciprocal social interactions of mice with DAT inhibition in SN neurons. a–c Experimental design illustrating assessments of habituation and following reciprocal social interactions (a). The amount of time engaged in facial sniffs (b) and anogenital sniffs (c) by mice injected with DAT-siRNA or control-siRNA is presented. Data are presented in box plots. * and ** denote the differences between the indicated groups at p < 0.05 and p < 0.01, respectively (Student’s t-test). (GIF 17 kb)
Supplementary Figure S2
Administration of SCH23390 in mice with DAT inhibition in SN neurons blocked autistic-like behaviors. a Experimental design and the time line for DAT-siRNA injection into the SN, administration of SCH23390 (0.02 mg/kg, i.p.) and following behavioral tests. b, c Representative tracks showing exploratory activity of mice injected with DAT-siRNA after SCH23390 or vehicle injection in the three chamber tests that aimed to measure sociability on choices between a social target and an empty cage (b), and social novelty exploration on choices between an earlier stranger (stranger 1) and a new stranger (stranger 2) (c). The amount of time the subject mouse spent in each chamber is presented. n = 9–12 animals. d–f Rearing (d), grooming (e), and digging (f) behavior of mice with DAT-siRNA injection and SCH23390 treatment. n = 8 animals, each. g, h Western blots showing ERK1/2, p-ERK1/2, CaMKIIα, p-CaMKIIα levels in the dorsal striatum of mice injected with DAT-siRNA after SCH23390 or vehicle injection (g). Quantification of Western blots (h). n = 6–8 animals, 3–4 repeats. Data are presented as mean ± SEM or box plots. * and ** denote the differences between the indicated groups at p < 0.05 and p < 0.01, respectively (Student’s t-test, two-way ANOVA, and Holm-Sidak post hoc test). (GIF 57 kb)
Supplementary Figure S3
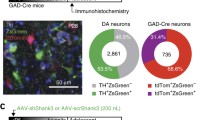
Optogenetic stimulation of the nigro-striatal circuit increases p-ERK1/2 and p-CaMKIIα levels in the dorsal striatum and induces repetitive behaviors. a Experimental design for optic stimulation of the nigro-striatal circuit (blue), repetitive behavior tests and following tissue sample preparation. Blue light (473 nm, at 20 Hz with a 30-ms spike width and a 3–3.5-mW light intensity) was administered for 5 min, as indicated. Tissue samples were prepared 60 min after optic stimulation (purple arrow). All examined animals had been used in the sociability tests (behavioral tests(a)), but had been in their home cages for more than 7 days prior to this experiment. A diagram illustrating the injection of viral vectors into the SN (green arrow) and optic stimulation of the nigro-striatal circuit through a fiber-optic (blue) (left panel). b, c Photomicrographs showing p-ERK1/2 and p-CaMKIIα expressions in the dorsal striatum of mice carrying AAV-CON (AAV-CON) or AAV-ChR2 (AAV-ChR2) following optic stimulation of the nigro-striatal circuit (b). Expression levels of p-ERK1/2- and p-CaMKIIα-positive cells were normalized to the control (c). Scale bars, 50 μm. n = 4 animals, each. d–f Rearing (d), grooming (e), and digging (f) of mice carrying AAV-ChR2 (AAV-ChR2) before (Off) and after (On) optic stimulation of the nigro-striatal circuit. n = 4 animals. Data are presented as mean ± SEM or box plots. * denotes the difference between the indicated groups at p < 0.05 (Student’s t-test). (GIF 71 kb)
Supplementary Figure S4
Administration of SKF38393 in normal mice induces autistic-like behaviors. a Experimental design for SKF38393 injection in normal mice, and following behavioral tests. b–d Representative tracks showing exploratory activity for sociability (b), social novelty exploration (c), and social preference (d) of normal mice treated with vehicle (Veh) or SKF38393 in the three-chamber test. The amount of time spent in each chamber is presented. n = 8–12 animals. e, f Reciprocal social interactions. The amount of time for facial sniff (e) and anogenital sniff (f) of mice treated with vehicle (Veh) or SKF38393 is presented. n = 9–10 animals. g–j Grooming (g), rearing (h), digging (i), and marble-burying (j) behaviors of mice injected with vehicle (Veh) or SKF38393 placed in a new cage. ** in Fig. S4j denotes the difference between vehicle and SKF38393 at p < 0.01 at the time points marked by arrowheads. n = 8–10. k, l Western blots showing p-ERK1/2, p-CaMKIIα, p-CREB, and p-GluN2B (S1303) levels in the dorsal striatum of mice injected with vehicle (Veh) or SKF38393 (k). Tissue samples were prepared 1 h after SKF38393 or vehicle injection. Quantification of Western blots (l). n = 4–6 animals, 4 repeats. Data are presented as mean ± SEM or box plots. * and ** denote the differences between the indicated groups at p < 0.05 and p < 0.01, respectively (Student’s t-test, two-way repeated measures ANOVA, two-way ANOVA, and Holm-Sidak post hoc test). (GIF 75 kb)
Supplementary Figure S5
D2 KO mice exhibit deficits in social behaviors and repetitive behaviors. a, b Representative tracks showing exploratory activity for social novelty exploration (a) and social preference (b) of WT and D2 KO mice in the three-chamber test. The amount of time the subject mouse spent in each chamber is presented. n = 10–19 animals. c, d Representative tracks showing exploratory activity for sociability (c) and social preference (d) of WT and D2 KO mice in the U-field assay. The amount of time the subject mouse spent in each field is presented. n = 9–10 animals. e–h Grooming (e), rearing (f), digging (g), and marble-burying behaviors (h) of WT and D2 KO mice when placed individually in a new cage. n = 9–12. i, j No sex difference in sociability (i) and social novelty exploration (j) of male and female D2 KO mice in the three-chamber test. The amount of time the subject mouse spent in each chamber is presented. n = 10 animals each. k–m No sex difference in grooming (k), rearing (l), and digging (m) of male and female D2 KO mice in a new cage. n = 7–8. n Olfactory habituation and dishabituation assays of WT and D2 KO mice. The amount of time of WT and D2 KO mice spent sniffing the cotton tip is presented. n = 6 animals each. Data are presented as mean ± SEM or box plots. * and ** denote the differences between the indicated groups at p < 0.05 and p < 0.01, respectively (Student’s t-test, two-way ANOVA, two-way repeated measures ANOVA, and Holm-Sidak post hoc test). For (n), * and ** denote the difference between previous odorant and indicated new odorant at p < 0.05 and p < 0.01. ## denotes the main effect of repeated treatment in indicated groups at p < 0.01 (two-way repeated measures ANOVA and Holm-Sidak post hoc test). (GIF 91 kb)
Supplementary Figure S6
Heterozygous D2 KO mice exhibit autistic-like behaviors following weak activation of D1 receptors. a, b Representative tracks showing exploratory activity for sociability (a) and social novelty exploration (b) of WT and heterozygous D1 KO mice (D1+/−) in the three chamber test. The amount of time the subject mouse spent in each chamber is presented. n = 11–13 animals each. c–e Grooming (c), rearing (d), and digging (e) of WT and heterozygous D1 KO mice placed in a new cage. n = 10–17. f, g Exploratory activity for sociability (f) and social novelty exploration (g) of WT and heterozygous D2 KO mice (D2+/−) in the three-chamber test. The amount of time the subject mouse spent in each chamber is presented. n = 10–11 animals each. h–j Grooming (h), rearing (i), and digging (j) of WT and heterozygous D2 KO mice placed in a new cage. n = 9–10. k, l Exploratory activity for sociability (k) and social novelty exploration (l) of WT and heterozygous D2 KO mice in the three-chamber test following SKF38393 treatment. The amount of time the subject mouse spent in each chamber is presented. Behaviors were examined 30 min after SKF38393 (0.5 mg/Kg, i.p.) injection. n = 7–8 animals. m–o Grooming (m), rearing (n), and digging (o) of WT and heterozygous D2 KO mice in a new cage 30 min after SKF38393 (0.5 mg/kg, i.p.) injection. n = 7–9 animals. Data are presented box plots. * and ** denote the differences between the indicated groups at p < 0.05 and p < 0.01, respectively (Student’s t-test, two-way ANOVA, and Holm-Sidak post hoc test). (GIF 82 kb)
Supplementary Figure S7
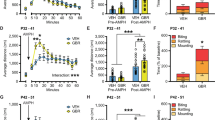
Analysis of physiological effects of increased p-CaMKIIα and p-ERK1/2, and transcript levels of synaptic proteins in the dorsal striatum of D2 KO mice. a Experimental design for CaMKIIα-siRNA or ERK2-siRNA injection in the dorsal striatum of D2 KO mice and following behavioral tests. b–e Sociability (b), grooming (c), rearing (d), and digging (e) of D2 KO mice with siRNA-mediated inhibition of CaMKIIα or ERK2 in the dorsal striatum. f–h Real-time PCR data showing transcript levels of D1 and D2 dopamine receptors (f), GluA1, GluA2, GluN1, GluN2A, and GluN2B glutamate receptor subunits (g), and the postsynaptic components of PSD93, PSD95, Homer1a, Shank3, and neuroligin 3(Ngn3) (h) in the dorsal striatum of WT and D2 KO mice. n = 6–8 animals, 4–8 repeats. Data are presented as box plots or mean ± SEM. * and ** denote the differences between the indicated groups at p < 0.05 and p < 0.01, respectively (Student’s t-test, one-way ANOVA, two-way ANOVA, and Holm-Sidak post hoc test). (GIF 56 kb)
Supplementary Figure S8
Analyses of the effective doses of ecopipam and SCH23390 for sociability and motor activity tests. a–g Timeline for drug injection and behavioral tests (a). Sociability (b, d, f) and locomotion (c, e, g) of D2 KO mice injected with ecopipam at the doses of 0.01–0.03 mg/kg. Ecopipam at the doses of 0.02–0.03 mg/kg (d–g), but not at 0.01 mg/kg (b, c), rescued sociability deficits (d, f) in the absence of motor activity suppression (e, g). h–p Sociability (h, j, m, o), locomotion (i, k, n, p) and grooming (l) of AC5 KO mice (h–n) or WT mice (o, p) injected with SCH23390 at the doses of 0.01–0.03 mg/kg. SCH23390 at the dose of 0.03 mg/kg rescued sociability deficits of AC5 KO mice (m) but produced motor activity suppression (n). Administration of SCH23390 (0.02 mg/kg) in WT mice did not change sociability (o) and locomotion (p). Data are presented as box plots (n = 6–8). * and ** denote the differences between the indicated groups at p < 0.05 and p < 0.01, respectively (Student’s t-test, two-way ANOVA, and Holm-Sidak post hoc test). (GIF 59 kb)
ESM 1
(XLSX 43 kb)
Rights and permissions
About this article
Cite this article
Lee, Y., Kim, H., Kim, JE. et al. Excessive D1 Dopamine Receptor Activation in the Dorsal Striatum Promotes Autistic-Like Behaviors. Mol Neurobiol 55, 5658–5671 (2018). https://doi.org/10.1007/s12035-017-0770-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0770-5