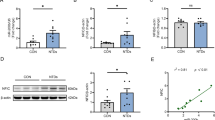
Abstract
Autophagy plays a very important role in numerous physiological and pathological events. However, it still remains unclear whether Atg7-induced autophagy is involved in the regulation of neural crest cell production. In this study, we found the co-location of Atg7 and Pax7+ neural crest cells in early chick embryo development. Upregulation of Atg7 with unilateral transfection of full-length Atg7 increased Pax7+ and HNK-1+ cephalic and trunk neural crest cell numbers compared to either Control-GFP transfection or opposite neural tubes, suggesting that Atg7 over-expression in neural tubes could enhance the production of neural crest cells. BMP4 in situ hybridization and p-Smad1/5/8 immunofluorescent staining demonstrated that upregulation of Atg7 in neural tubes suppressed the BMP4/Smad signaling, which is considered to promote the delamination of neural crest cells. Interestingly, upregulation of Atg7 in neural tubes could significantly accelerate cell progression into the S phase, implying that Atg7 modulates cell cycle progression. However, β-catenin expression was not significantly altered. Finally, we demonstrated that upregulation of the Atg7 gene could activate autophagy as did Atg8. We have also observed that similar phenotypes, such as more HNK-1+ neural crest cells in the unilateral Atg8 transfection side of neural tubes, and the transfection with full-length Atg8-GFP certainly promote the numbers of BrdU+ neural crest cells in comparison to the GFP control. Taken together, we reveal that Atg7-induced autophagy is involved in regulating the production of neural crest cells in early chick embryos through the modification of the cell cycle.







Similar content being viewed by others
References
Aburto MR, Sanchez-Calderon H, Hurle JM et al (2012) Early otic development depends on autophagy for apoptotic cell clearance and neural differentiation. Cell Death Dis 3:e394
Qu X, Zou Z, Sun Q et al (2007) Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell 128:931–946
Mellen MA, de la Rosa EJ, Boya P (2008) The autophagic machinery is necessary for removal of cell corpses from the developing retinal neuroepithelium. Cell Death Differ 15:1279–1290
Ravikumar B, Sarkar S, Davies JE et al (2010) Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 90:1383–1435
Gallagher LE, Williamson LE, Chan EY (2016) Advances in autophagy regulatory mechanisms. Cells 5
Russell RC, Yuan HX, Guan KL (2014) Autophagy regulation by nutrient signaling. Cell Res 24:42–57
Kuma A, Hatano M, Matsui M et al (2004) The role of autophagy during the early neonatal starvation period. Nature 432:1032–1036
Komatsu M, Waguri S, Ueno T et al (2005) Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 169:425–434
Hall BK (2008) The neural crest and neural crest cells: discovery and significance for theories of embryonic organization. J Biosci 33:781–793
Cordero DR, Brugmann S, Chu Y et al (2011) Cranial neural crest cells on the move: their roles in craniofacial development. Am J Med Genet A 155A:270–279
Schneider RA (1999) Neural crest can form cartilages normally derived from mesoderm during development of the avian head skeleton. Dev Biol 208:441–455
Pinsky L (1976) Letter: neural-crest injury and congenital malformations. Lancet 1:637
McCredie J, Cameron J, Shoobridge R (1978) Congenital malformations and the neural crest. Lancet 2:761–763
Gale TF, Kirby ML (1996) Absence of correlation between transient cranial hemorrhages and congenital malformations following neural crest ablation in chicks. Teratology 53:318–325
Basch ML, Bronner-Fraser M (2006) Neural crest inducing signals. Adv Exp Med Biol 589:24–31
Coucouvanis E, Martin GR (1999) BMP signaling plays a role in visceral endoderm differentiation and cavitation in the early mouse embryo. Development 126:535–546
Levine B, Yuan J (2005) Autophagy in cell death: an innocent convict? J Clin Invest 115:2679–2688
Liu Y, Schiff M, Czymmek K et al (2005) Autophagy regulates programmed cell death during the plant innate immune response. Cell 121:567–577
Wang G, Li Y, Wang XY et al (2015) Misexpression of BRE gene in the developing chick neural tube affects neurulation and somitogenesis. Mol Biol Cell 26:978–992
Wang G, Li Y, Wang XY et al (2013) Slit/Robo1 signaling regulates neural tube development by balancing neuroepithelial cell proliferation and differentiation. Exp Cell Res 319:1083–1093
Lee IH, Kawai Y, Fergusson MM et al (2012) Atg7 modulates p53 activity to regulate cell cycle and survival during metabolic stress. Science 336:225–228
Yang X, Dormann D, Munsterberg AE et al (2002) Cell movement patterns during gastrulation in the chick are controlled by positive and negative chemotaxis mediated by FGF4 and FGF8. Dev Cell 3:425–437
Chapman SC, Collignon J, Schoenwolf GC et al (2001) Improved method for chick whole-embryo culture using a filter paper carrier. Dev Dyn 220:284–289
Yang X, Chrisman H, Weijer CJ (2008) PDGF signalling controls the migration of mesoderm cells during chick gastrulation by regulating N-cadherin expression. Development 135:3521–3530
Yue Q, Wagstaff L, Yang X et al (2008) Wnt3a-mediated chemorepulsion controls movement patterns of cardiac progenitors and requires RhoA function. Development 135:1029–1037
Somi S, Buffing AA, Moorman AF et al (2004) Dynamic patterns of expression of BMP isoforms 2, 4, 5, 6, and 7 during chicken heart development. Anat Rec A Discov Mol Cell Evol Biol 279:636–651
Henrique D, Adam J, Myat A et al (1995) Expression of a Delta homologue in prospective neurons in the chick. Nature 375:787–790
Cayuso J, Ulloa F, Cox B et al (2006) The Sonic hedgehog pathway independently controls the patterning, proliferation and survival of neuroepithelial cells by regulating Gli activity. Development 133:517–528
Lacosta AM, Muniesa P, Ruberte J et al (2005) Novel expression patterns of Pax3/Pax7 in early trunk neural crest and its melanocyte and non-melanocyte lineages in amniote embryos. Pigment Cell Res 18:243–251
Wu X, Howard MJ (2001) Two signal transduction pathways involved in the catecholaminergic differentiation of avian neural crest-derived cells in vitro. Mol Cell Neurosci 18:394–406
Hari L, Miescher I, Shakhova O et al (2012) Temporal control of neural crest lineage generation by Wnt/beta-catenin signaling. Development 139:2107–2117
Wang XY, Li S, Wang G et al (2015) High glucose environment inhibits cranial neural crest survival by activating excessive autophagy in the chick embryo. Sci Rep 5:18321
Fraker PJ (2005) Roles for cell death in zinc deficiency. J Nutr 135:359–362
Juhasz G, Erdi B, Sass M et al (2007) Atg7-dependent autophagy promotes neuronal health, stress tolerance, and longevity but is dispensable for metamorphosis in Drosophila. Genes Dev 21:3061–3066
Simkin JE, Zhang D, Ighaniyan S et al (2014) Parameters affecting efficiency of in ovo electroporation of the avian neural tube and crest. Dev Dyn 243:1440–1447
Lamalice L, Le Boeuf F, Huot J (2007) Endothelial cell migration during angiogenesis. Circ Res 100:782–794
Zhuo C, Ji Y, Chen Z et al (2013) Proteomics analysis of autophagy-deficient Atg7−/− MEFs reveals a close relationship between F-actin and autophagy. Biochem Biophys Res Commun 437:482–488
Chalpe AJ, Prasad M, Henke AJ et al (2010) Regulation of cadherin expression in the chicken neural crest by the Wnt/beta-catenin signaling pathway. Cell Adhes Migr 4:431–438
Hegarty SV, O'Keeffe GW, Sullivan AM (2013) BMP-Smad 1/5/8 signalling in the development of the nervous system. Prog Neurobiol 109:28–41
Oyedele OO, Kramer B (2013) Nuanced but significant: how ethanol perturbs avian cranial neural crest cell actin cytoskeleton, migration and proliferation. Alcohol 47:417–426
Carter TC, Kay DM, Browne ML et al (2012) Hirschsprung’s disease and variants in genes that regulate enteric neural crest cell proliferation, migration and differentiation. J Hum Genet 57:485–493
Gao C, Cao W, Bao L et al (2010) Autophagy negatively regulates Wnt signalling by promoting dishevelled degradation. Nat Cell Biol 12:781–790
Jia Z, Wang J, Wang W et al (2014) Autophagy eliminates cytoplasmic beta-catenin and NICD to promote the cardiac differentiation of P19CL6 cells. Cell Signal 26:2299–2305
Jang J, Wang Y, Lalli MA et al (2016) Primary cilium-autophagy-Nrf2 (PAN) axis activation commits human embryonic stem cells to a neuroectoderm fate. Cell 165:410–420
Lu WH, Wang G, Li Y et al (2014) Autophagy functions on EMT in gastrulation of avian embryo. Cell Cycle 13:2752–2764
Acknowledgements
We thank Maurice van den Hoff for the BMP4 plasmid. This study was supported by NSFC grant (81571436, 31401230), Science and Technology Planning Project of Guangdong Province (2016B030229002), Science and Technology Program of Guangzhou (201710010054, 201510010073), Guangdong Natural Science Foundation (2016A030311044), Research Grant of Key Laboratory of Regenerative Medicine, Ministry of Education, Jinan University (Nos. ZSYX-M-00001 and ZSYX-T-00001), Fund for Science and Technology Innovation of Guangdong College Student (pdjh 2017b060) and Students Research Training Program Fund (CX16014, 16112011).
Author information
Authors and Affiliations
Contributions
G.W., E.C., S.C., C.L., J.L., and L.G. performed the experiments and collected the data; G.W., M.C., A.M., L.C., and X. Y. designed the study and analyzed the data; and Y.B. and X.Y. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Guang Wang, En-ni Chen, and Chang Liang contribute to the work equally.
Electronic supplementary material
Fig. S1
Atg7 expression following the over-expression of Atg7 on neural tubes. Half side of HH10 chick neural tubes was transfected with FL-Atg7, and then the transfected embryos were further incubated for 10 h before Atg7 immunofluorescent staining was implemented and sectioned. A-D: The representative transverse sections of DAPI staining (A), autofluorescence (B), Atg7 staining (C) and the merge (D) of A and C. Scale bars = 50 μm in A-D. (GIF 131 kb)
Fig. S2
Assessment of HNK-1 + neural crest production following the over-expression of Atg7 on neural tubes. Half side of HH10 chick neural tubes was transfected with FL-Atg7 + GFP, and then the transfected embryos were further incubated for 10 h before HNK-1 immunofluorescent staining was implemented and sectioned. A-C: The representative transverse sections of FL-Atg7 + GFP transfection (A), HNK-1 staining (B) and the merge (C) of A and B at cranial level. A1-C1: The high magnification images from the sites indicated by dotted squares in A-C respectively. D-F: The representative transverse sections of FL-Atg7 + GFP transfection (D), HNK-1 staining (E) and the merge (F) of D and E at cardiac level. D1-F1: The high magnification images from the sites indicated by dotted squares in D-F respectively. G-H: The bar charts show the comparisons of the HNK-1+ areas between the transfected side and opposite control side. Abbreviations: NT, neural tube; HT, heart tube. Scale bars = 100 μm in A-F and 50 μm in A1-F1. (GIF 158 kb)
Fig. S3
Polarization and protrusion formation were changed following the up-regulation of Atg7 on NCCs. After transfection of neural tubes with either FL-Atg7 plasmids, the neural tubes were isolated and cultured to allow the NCCs to migrate out of the tubes. A-D: The representative images of cells, in which were stained with DAPI (A), Atg7 (B), F-actin (C), and (D) was merged image. E-F: Bar charts showing the ratio of long axis to short axis (E) and the numbers of the antennas (F). Scale bars = 20 μm in A-D. (GIF 78 kb)
Fig. S4
Assessment of HNK-1 + and Pax7 + neural crest production following the down-regulation of Atg7 on neural tubes. Half side of HH10 chick neural tubes was transfected with Mo-Atg7 + GFP, and then the transfected embryos were further incubated for 10 h before HNK-1 or Pax7 immunofluorescent staining was implemented and sectioned. A-C: The representative transverse sections of Mo-Atg7 + GFP transfection (A), Pax7 staining (B) and the merge (C) of A and B. DAPI stains in C. D-F: The representative transverse sections of Mo-Atg7 + GFP transfection (D), HNK-1 staining (E) and the merge (F) of D and E. DAPI stains in F. G-H: The bar charts show the comparisons of the Pax7+ cell numbers (G) and HNK-1+ areas (H) between the transfected side and opposite control side. Abbreviations: Mo-Atg7, morpholino-Atg7. Scale bars = 50 μm in A-F. (GIF 86 kb)
Fig. S5
Over-expressing Atg7 and Atg8 increased the numbers of BrdU positive cells at neural tubes. Half side of HH10 chick neural tubes was transfected with either Control GFP, FL-Atg7 + GFP or FL-Atg8-GFP, and then the transfected embryos were further incubated for 10 h before BrdU immunofluorescent staining was implemented. A-C: The representative transverse sections of GFP transfection (A), BrdU staining (B) and merge (C) of A and B from Control-GFP transfected chick embryo group. D-F: The representative transverse sections of FL-Atg7 + GFP transfection (D), BrdU staining (E) and merge (F) of D and E from FL-Atg7 + GFP transfected chick embryo group. G-I: The representative transverse sections of FL-Atg8-GFP transfection (G), BrdU staining (H) and merge (I) of G and H from FL-Atg8-GFP transfected chick embryo group. J-K: The bar chart shows the ratios of BrdU+ neural crest cell number in transfected side and BrdU+ neural crest cell numbers in opposite control side between control and FL-Atg7 + GFP or FL-Atg8-GFP transfection groups. Scale bars = 20 μm in A-I. (GIF 123 kb)
Fig. S6
Assessment of Atg8 expression following the over-regulation of Atg8 on neural tubes. Half side of HH10 chick neural tubes was transfected with either Control GFP or FL-Atg8-GFP, and then the transfected embryos were further incubated for 10 h before Atg8 immunofluorescent staining was implemented and sectioned. A-C: The representative transverse sections of Control-GFP transfection (A), Atg8 staining (B) and the merge (C) of A and B from Control-GFP transfected embryos. DAPI stains in C. D-F: The representative transverse sections of FL-Atg8-GFP transfection (D), Atg8 staining (E) and the merge (F) of D and E from FL-Atg8-GFP transfected embryos. DAPI stains in F. Scale bars = 50 μm in A-F. (GIF 192 kb)
Rights and permissions
About this article
Cite this article
Wang, G., Chen, En., Liang, C. et al. Atg7-Mediated Autophagy Is Involved in the Neural Crest Cell Generation in Chick Embryo. Mol Neurobiol 55, 3523–3536 (2018). https://doi.org/10.1007/s12035-017-0583-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0583-6