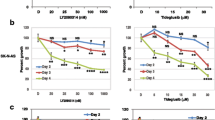
Abstract
Neuroblastoma (NB) is an extracranial solid cancer and the most common cancer in infancy. Despite the standard treatment for NB is based on the combination of chemotherapeutic drugs such as doxorubicin, vincristine, cyclophosphamide, and cisplatin, chemoresistance occurs over the time. The aim of the present research was to evaluate the effect of bortezomib (BTZ) (50 nM) on NB cell viability and how lipoic acid (ALA) (100 μM) modifies pharmacological response to this chemotherapeutic agent. Cell viability was assessed by ATP luminescence assay whereas expression of oxidative stress marker (i.e., heme oxygenase-1) and endoplasmic reticulum stress proteins was performed by real-time PCR, western blot, and immunofluorescence. Our data showed that BTZ treatment significantly reduced cell viability when compared to untreated cultures (about 40%). Interestingly, ALA significantly reduced the efficacy of BTZ (about 30%). Furthermore, BTZ significantly induced heme oxygenase-1 as a result of increased oxidative stress and such overexpression was prevented by concomitant treatment with ALA. Similarly, ALA significantly reduced BTZ-mediated endoplasmic reticulum stress as measured by reduction in BiP1 and IRE1α, ERO1α, and PDI expression. In conclusion, our data suggest that BTZ efficacy is dependent on cellular redox status and such mechanisms may be responsible of chemoresistance to this chemotherapeutic agent.





Similar content being viewed by others
References
Park JR, Eggert A, Caron H (2010) Neuroblastoma: biology, prognosis, and treatment. Hematol Oncol Clin North Am 24(1):65–86. doi:10.1016/j.hoc.2009.11.011
Ohira M, Nakagawara A (2010) Global genomic and RNA profiles for novel risk stratification of neuroblastoma. Cancer Sci 101(11):2295–2301. doi:10.1111/j.1349-7006.2010.01681.x
Romano A, Conticello C, Di Raimondo F (2013) Bortezomib for the treatment of previously untreated multiple myeloma. Immunotherapy 5(4):327–352. doi:10.2217/imt.13.14
Mujtaba T, Dou QP (2011) Advances in the understanding of mechanisms and therapeutic use of bortezomib. Discov Med 12(67):471–480
Du BY, Song W, Bai L, Shen Y, Miao SY, Wang LF (2012) Synergistic effects of combination treatment with bortezomib and doxorubicin in human neuroblastoma cell lines. Chemotherapy 58(1):44–51. doi:10.1159/000335603
Furfaro AL, Piras S, Passalacqua M, Domenicotti C, Parodi A, Fenoglio D, Pronzato MA, Marinari UM et al (2014) HO-1 up-regulation: a key point in high-risk neuroblastoma resistance to bortezomib. Biochim Biophys Acta 1842(4):613–622. doi:10.1016/j.bbadis.2013.12.008
Tibullo D, Barbagallo I, Giallongo C, Vanella L, Conticello C, Romano A, Saccone S, Godos J et al (2016) Heme oxygenase-1 nuclear translocation regulates bortezomibinduced cytotoxicity and mediates genomic instability in myeloma cells. Oncotarget. doi:10.18632/oncotarget.7563
Ryter SW, Choi AM (2009) Heme oxygenase-1/carbon monoxide: from metabolism to molecular therapy. Am J Respir Cell Mol Biol 41(3):251–260. doi:10.1165/rcmb.2009-0170TR
Goswami B, Rajappa M, Sharma M, Sharma A (2008) Inflammation: its role and interplay in the development of cancer, with special focus on gynecological malignancies. Int J Gynecol Cancer 18(4):591–599. doi:10.1111/j.1525-1438.2007.01089.x
Abe M (2011) Guest editorial: understanding the pathogenesis and the evolving treatment paradigm for multiple myeloma in the era of novel agents. Int J Hematol 94(4):307–309. doi:10.1007/s12185-011-0950-4
Meister S, Schubert U, Neubert K, Herrmann K, Burger R, Gramatzki M, Hahn S, Schreiber S et al (2007) Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res 67(4):1783–1792. doi:10.1158/0008-5472.CAN-06-2258
Teicher BA, Ara G, Herbst R, Palombella VJ, Adams J (1999) The proteasome inhibitor PS-341 in cancer therapy. Clin Cancer Res 5(9):2638–2645
Richardson PG, Baz R, Wang M, Jakubowiak AJ, Laubach JP, Harvey RD, Talpaz M, Berg D et al (2014) Phase 1 study of twice-weekly ixazomib, an oral proteasome inhibitor, in relapsed/refractory multiple myeloma patients. Blood 124(7):1038–1046. doi:10.1182/blood-2014-01-548826
Salerno L, Pittala V, Romeo G, Modica MN, Siracusa MA, Di Giacomo C, Acquaviva R, Barbagallo I et al (2013) Evaluation of novel aryloxyalkyl derivatives of imidazole and 1,2,4-triazole as heme oxygenase-1 (HO-1) inhibitors and their antitumor properties. Bioorg Med Chem 21(17):5145–5153. doi:10.1016/j.bmc.2013.06.040
Goodman AI, Choudhury M, da Silva JL, Schwartzman ML, Abraham NG (1997) Overexpression of the heme oxygenase gene in renal cell carcinoma. Proc Soc Exp Biol Med 214(1):54–61
Becker JC, Fukui H, Imai Y, Sekikawa A, Kimura T, Yamagishi H, Yoshitake N, Pohle T et al (2007) Colonic expression of heme oxygenase-1 is associated with a better long-term survival in patients with colorectal cancer. Scand J Gastroenterol 42(7):852–858. doi:10.1080/00365520701192383
Yamada T, Hashida K, Takarada-Iemata M, Matsugo S, Hori O (2011) Alpha-lipoic acid (LA) enantiomers protect SH-SY5Y cells against glutathione depletion. Neurochem Int 59(7):1003–1009. doi:10.1016/j.neuint.2011.09.005
Bramanti V, Tomassoni D, Bronzi D, Grasso S, Curro M, Avitabile M, Li Volsi G, Renis M et al (2010) Alpha-lipoic acid modulates GFAP, vimentin, nestin, cyclin D1 and MAP-kinase expression in astroglial cell cultures. Neurochem Res 35(12):2070–2077. doi:10.1007/s11064-010-0256-6
Giallongo C, Parrinello N, Tibullo D, La Cava P, Romano A, Chiarenza A, Barbagallo I, Palumbo GA et al (2014) Myeloid derived suppressor cells (MDSCs) are increased and exert immunosuppressive activity together with polymorphonuclear leukocytes (PMNs) in chronic myeloid leukemia patients. PLoS One 9(7):e101848. doi:10.1371/journal.pone.0101848
Giallongo C, Tibullo D, La Cava P, Branca A, Parrinello N, Spina P, Stagno F, Conticello C et al (2011) BRIT1/MCPH1 expression in chronic myeloid leukemia and its regulation of the G2/M checkpoint. Acta Haematol 126(4):205–210. doi:10.1159/000329911
Anfuso CD, Motta C, Giurdanella G, Arena V, Alberghina M, Lupo G (2014) Endothelial PKCalpha-MAPK/ERK-phospholipase A2 pathway activation as a response of glioma in a triple culture model. A new role for pericytes? Biochimie 99:77–87. doi:10.1016/j.biochi.2013.11.013
Avola R, Di Tullio MA, Fisichella A, Tayebati SK, Tomassoni D (2004) Glial fibrillary acidic protein and vimentin expression is regulated by glucocorticoids and neurotrophic factors in primary rat astroglial cultures. Clin Exp Hypertens 26(4):323–333
Bramanti V, Bronzi D, Tomassoni D, Li Volti G, Cannavo G, Raciti G, Napoli M, Vanella A et al (2008) Effect of choline-containing phospholipids on transglutaminase activity in primary astroglial cell cultures. Clin Exp Hypertens 30(8):798–807. doi:10.1080/10641960802563576
Bramanti V, Grasso S, Tomassoni D, Traini E, Raciti G, Viola M, Li Volti G, Campisi A et al (2015) Effect of growth factors and steroid hormones on heme oxygenase and cyclin D1 expression in primary astroglial cell cultures. J Neurosci Res 93(3):521–529. doi:10.1002/jnr.23506
Scuderi MR, Anfuso CD, Lupo G, Motta C, Romeo L, Guerra L, Cappellani A, Ragusa N et al (2008) Expression of Ca(2+)-independent and Ca(2+)-dependent phospholipases A(2) and cyclooxygenases in human melanocytes and malignant melanoma cell lines. Biochim Biophys Acta 1781(10):635–642. doi:10.1016/j.bbalip.2008.07.007
Lupo G, Anfuso CD, Ragusa N, Tirolo C, Marchetti B, Gili E, La Rosa C, Vancheri C (2007) Activation of cytosolic phospholipase A2 and 15-lipoxygenase by oxidized low-density lipoproteins in cultured human lung fibroblasts. Biochim Biophys Acta 1771(4):522–532. doi:10.1016/j.bbalip.2007.01.014
Bramanti V, Tomassoni D, Grasso S, Bronzi D, Napoli M, Campisi A, Li Volti G, Ientile R et al (2012) Cholinergic precursors modulate the expression of heme oxigenase-1, p21 during astroglial cell proliferation and differentiation in culture. Neurochem Res 37(12):2795–2804. doi:10.1007/s11064-012-0873-3
Giurdanella G, Anfuso CD, Olivieri M, Lupo G, Caporarello N, Eandi CM, Drago F, Bucolo C et al (2015) Aflibercept, bevacizumab and ranibizumab prevent glucose-induced damage in human retinal pericytes in vitro, through a PLA2/COX-2/VEGF-A pathway. Biochem Pharmacol 96(3):278–287. doi:10.1016/j.bcp.2015.05.017
Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, Munshi N, Dang L et al (2002) NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem 277(19):16639–16647. doi:10.1074/jbc.M200360200
Almond JB, Cohen GM (2002) The proteasome: a novel target for cancer chemotherapy. Leukemia 16(4):433–443. doi:10.1038/sj.leu.2402417
Obeng EA, Carlson LM, Gutman DM, Harrington WJ Jr, Lee KP, Boise LH (2006) Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 107(12):4907–4916. doi:10.1182/blood-2005-08-3531
Chien W, Ding LW, Sun QY, Torres-Fernandez LA, Tan SZ, Xiao J, Lim SL, Garg M et al (2014) Selective inhibition of unfolded protein response induces apoptosis in pancreatic cancer cells. Oncotarget 5(13):4881–4894. doi:10.18632/oncotarget.2051
Furfaro AL, Piras S, Domenicotti C, Fenoglio D, De Luigi A, Salmona M, Moretta L, Marinari UM et al (2016) Role of Nrf2, HO-1 and GSH in neuroblastoma cell resistance to bortezomib. PLoS One 11(3):e0152465. doi:10.1371/journal.pone.0152465
Ghobrial IM, Munshi NC, Harris BN, Shi P, Porter NM, Schlossman RL, Laubach JP, Anderson KC et al (2011) A phase I safety study of enzastaurin plus bortezomib in the treatment of relapsed or refractory multiple myeloma. Am J Hematol 86(7):573–578. doi:10.1002/ajh.22048
Li Volti G, Ientile R, Abraham NG, Vanella A, Cannavo G, Mazza F, Curro M, Raciti G et al (2004) Immunocytochemical localization and expression of heme oxygenase-1 in primary astroglial cell cultures during differentiation: effect of glutamate. Biochem Biophys Res Commun 315(2):517–524. doi:10.1016/j.bbrc.2004.01.090
Lin Q, Weis S, Yang G, Weng YH, Helston R, Rish K, Smith A, Bordner J et al (2007) Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem 282(28):20621–20633. doi:10.1074/jbc.M607954200
Tibullo D, Barbagallo I, Giallongo C, La Cava P, Parrinello N, Vanella L, Stagno F, Palumbo GA et al (2013) Nuclear translocation of heme oxygenase-1 confers resistance to imatinib in chronic myeloid leukemia cells. Curr Pharm Des 19(15):2765–2770
Gandini NA, Fermento ME, Salomon DG, Blasco J, Patel V, Gutkind JS, Molinolo AA, Facchinetti MM et al (2012) Nuclear localization of heme oxygenase-1 is associated with tumor progression of head and neck squamous cell carcinomas. Exp Mol Pathol 93(2):237–245. doi:10.1016/j.yexmp.2012.05.001
Biswas C, Shah N, Muthu M, La P, Fernando AP, Sengupta S, Yang G, Dennery PA (2014) Nuclear heme oxygenase-1 (HO-1) modulates subcellular distribution and activation of Nrf2, impacting metabolic and anti-oxidant defenses. J Biol Chem 289(39):26882–26894. doi:10.1074/jbc.M114.567685
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tibullo, D., Giallongo, C., Puglisi, F. et al. Effect of Lipoic Acid on the Biochemical Mechanisms of Resistance to Bortezomib in SH-SY5Y Neuroblastoma Cells. Mol Neurobiol 55, 3344–3350 (2018). https://doi.org/10.1007/s12035-017-0575-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0575-6