Abstract
Parkinson’s disease (PD) is characterized by the appearance of motor symptoms many years after the onset of neurodegeneration, which explains low efficiency of therapy. Therefore, one of the priorities in neurology is to develop an early diagnosis and preventive treatment of PD, based on knowledge of molecular mechanisms of neurodegeneration and neuroplasticity in the nigrostriatal system. However, due to inability to diagnose PD at preclinical stage, research and development must be performed in animal models by comparing the nigrostriatal system in the models of asymptomatic and early symptomatic stages of PD. In this study, we showed that despite the progressive loss of neurons in the substantia nigra at the presymptomatic and symptomatic stage, almost no change was observed in the main functional characteristics of this brain region, including dopamine (DA) uptake and release, dopamine transporter (DAT) and vesicular monoamine transporter 2 (VMAT2) expression, and activity of MAO-A and MAO-B. In the striatum of presymptomatic mice, some parameters (DA release and uptake, MAO-A activity) remained compensatory unchanged or compensatory decreased (MAO-B gene expression and activity), while others—a reduction in DA levels in tissue and extracellular space and in VMAT2 and DAT expression—manifest the functional failure. In symptomatic mice, only a few parameters (spontaneous DA release and uptake, MAO-B gene expression and activity) remained at the same level as at presymptomatic stage, while most parameters (DA level in tissue and extracellular space, DA-stimulated release, VMAT2 and DAT contents), decreased, showing decompensation, which was enhanced by increasing MAO-A activity. Thus, this study provides a comprehensive assessment of the molecular mechanisms of neuroplasticity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine models of preclinical and clinical stages of PD, which could potentially serve as a powerful tool for translational medicine.













Similar content being viewed by others
References
Bernheimer H, Birkmayer W, Hornykiewicz O et al (1973) Brain dopamine and the syndromes of Parkinson and Huntington clinical, morphological and neurochemical correlations. J Neurol Sci 20:415–455
Riederer P, Wuketich S (1976) Time course of nigrostriatal degeneration in Parkinson’s disease. A detailed study of influential factors in human brain amine analysis. J Neural Transm 38:277–301
Bezard E, Gross CE (1998) Compensatory mechanisms in experimental and human parkinsonism: towards a dynamic approach. Prog Neurobiol 55:93–116
Zigmond MJ (1997) Do compensatory processes underlie the preclinical phase of neurodegenerative disease? Insights from an animal model of parkinsonism. Neurobiol Dis 4:247–253
Bergstrom BP, Garris PA (2003) “Passive stabilization” of striatal extracellular dopamine across the lesion spectrum encompassing the presymptomatic phase of Parkinson’s disease: a voltammetric study in the 6-OHDA-lesioned rat. J Neurochem 87:1224–1236
Bergstrom BP, Sanberg SG, Andersson M et al (2011) Functional reorganization of the presynaptic dopaminergic terminal in parkinsonism. Neuroscience 193:310–322
Ugrumov MV (2008) Brain neurons partly expressing monoaminergic phenotype: distribution, development, and functional significance in norm and pathology. In: Lajtha A, Vizi ES (eds) Handb. Neurochem. Mol. Neurobiol. Springer, US, pp 21–73
Blesa J, Pifl C, Sánchez-González MA, Juri C et al (2012) The nigrostriatal system in the presymptomatic and symptomatic stages in the MPTP monkey model: a PET, histological and biochemical study. Neurobiol Dis 481:79–91
Sharma S, Moon CS, Khogali A et al (2013) Biomarkers in Parkinson’s disease (recent update). NeurochemInt 63:201–229
Miller GW, Erickson JD, Perez JT et al (1999) Immunochemical analysis of vesicular monoamine transporter (VMAT2) protein in Parkinson’s disease. ExpNeurol 156:138–148
Bezard E, Dovero S, Prunier C et al (2001) Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J Neurosci 21:6853–6861
Carrillo MC, Brashear HR, Logovinsky V et al (2013) Can we prevent Alzheimer’s disease? Secondary “prevention” trials in Alzheimer’s disease. Alzheimers Dement 9:123–131
Yun JW, Ahn JB, Kang BC (2015) Modeling Parkinson’s disease in the common marmoset (Callithrixjacchus): overview of models, methods, and animal care. Lab Anim Res 31:155–165
Berendse HW, Booij J, Francot CM et al (2001) Subclinical dopaminergic dysfunction in asymptomatic Parkinson’s disease patients’ relatives with a decreased sense of smell. Ann Neurol 50:34–41
Goldstein DS (2003) Imaging of the autonomic nervous system: focus on cardiac sympathetic innervation. Semin Neurol 23:423–433
Perlmutter JS, Norris SA (2014) Neuroimaging biomarkers for Parkinson disease: facts and fantasy. Ann Neurol 76:769–783
DeKosky ST, Marek K (2003) Looking backward to move forward: early detection of neurodegenerative disorders. Science 302:830–834
Eller M, Williams DR (2009) Biological fluid biomarkers in neurodegenerative parkinsonism. Nat Rev Neurol 5:561–570
Perkin GD (1981) Autonomic function. In: Rose FC, Capildeo R (eds) Research progress in Parkinson’s disease. Pitman Books, London, pp. 111–125
Sandyk R, Iacono RP, Bamford CR (1987) The hypothalamus in Parkinson disease. Ital J Neurol Sci 8:227–234
Halliday GM, Blumbergs PC, Cotton RGH et al (1990) Loss of brainstem serotonin-and substance P-containing neurons in Parkinson’s disease. Brain Res 510:104–107
Jellinger KA (1991) Pathology of Parkinson’s disease. Mol Chem Neuropathol 14:153–197
Hague K, Lento P, Morgello S et al (1997) The distribution of Lewy bodies in pure autonomic failure: autopsy findings and review of the literature. Acta Neuropathol 94:192–196
Goldstein DS, Holmes C, Li ST et al (2000) Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med 133:338–347
Braak H, Ghebremedhin E, Rüb U et al (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318:121–134
Klos KJ, Ahlskog JE, Josephs KA et al (2006) α-Synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology 66:1100–1102
Petrucelli L, Dickson DW (2008) Neuropathology of Parkinson’s disease. In: Nass R, Przedborski S (eds) Parkinson’s disease: molecular and therapeutic insights from model systems. Elsevier, Amsterdam, pp. 35–48
Akhtar RS, Stern MB (2012) New concepts in the early and preclinical detection of Parkinson’s disease: therapeutic implications. Expert Rev Neurother 12:1429–1438
Bezard E, Yue Z, Kirik D, Spillantini MG (2013) Animal models of Parkinson’s disease: limits and relevance to neuroprotection studies. Mov Disorders 28:61–70
Chen H, Burton EA, Ross GW et al (2013) Research on the premotor symptoms of Parkinson’s disease: clinical and etiological implications. Environ Health Perspect 121:1245–1252
Mahlknecht P, Poewe W (2013) Is there a need to redefine Parkinson’s disease? J Neural Transm 120:9–17
Langston JW (2006) The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol 59:591–596
Goldstein DS, Sewell L (2009) Olfactory dysfunction in pure autonomic failure: implications for the pathogenesis of Lewy body diseases. Parkinsonism Relat Disord 15:516–520
Purisai MG, McCormack AL, Langston WJ et al (2005) α-Synuclein expression in the substantia nigra of MPTP-lesioned non-human primates. Neurobiol Dis 20:898–906
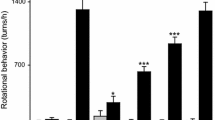
Kozina EA, Khakimova GR, Khaindrava VG et al (2014) Tyrosine hydroxylase expression and activity in nigrostriatal dopaminergic neurons of MPTP-treated mice at the presymptomatic and symptomatic stages of parkinsonism. J NeurolSci 340:198–207
Halliday G, Herrero MT, Murphy K et al (2009) No Lewy pathology in monkeys with over 10 years of severe MPTP Parkinsonism. MovDisord 24:1519–1523
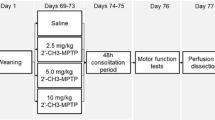
Ugrumov MV, Khaindrava VG, Kozina EA et al (2011) Modeling of presymptomatic and symptomatic stages of parkinsonism in mice. Neuroscience 181:175–188
Kozina EA, Kim AR, Kurina AY, Ugrumov MV (2016) Cooperative synthesis of dopamine by non-dopaminergic neurons as a compensatory mechanism in the striatum of mice with MPTP-induced Parkinsonism. Neurobiol Dis 98:108–121
Alieva AK, Filatova EV, Kolacheva AA et al (2016) Transcriptome profile changes in mice with MPTP-induced early stages of Parkinson’s disease. MolNeurobiol. doi:10.1007/s12035-016-0190-y
Simunovic F, Yi M, Wang Y et al (2009) Gene expression profiling of substantia nigra dopamine neurons: further insights into Parkinson’s disease pathology. Brain 132:1795–1809
Luthman J, Jonsson G (1986) Effects of the parkinsonism-inducing neurotoxin MPTP and its metabolite MPP+ on sympathetic adrenergic nerves in mouse iris and atrium. Medical biology 64:95–102
Chaumette T, Lebouvier T, Aubert P et al (2009) Neurochemical plasticity in the enteric nervous system of a primate animal model of experimental Parkinsonism. Neurogastroenterol Motility 21:215–222
Potts LF, Wu H, Singh A et al (2014) Modeling Parkinson’s disease in monkeys for translational studies, a critical analysis. ExpNeurol 256:133–143
Markey SP, Johannessen JN, Chiueh CC et al (1984) Intraneuronal generation of a pyridinium metabolite may cause drug-induced parkinsonism. Nature 311:464–467
Nakazato T, Akiyama A (1998) Differential time courses of exogenous 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine and its metabolite MPP+ in the rat striatum and nucleus accumbens measured using in vivo voltammetry. Brain Res 812:150–156
Paxinos G, Franklin K (2012) Paxinos and Franklin’s the mouse brain in stereotaxic coordinates, 4th edn. Elsevier/Academic Press, Amsterdam
Smith P, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano M, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ (2008) The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods 172:250–254
Romero-Calvo I, Ocón B, Martínez-Moya P, Suárez MD, Zarzuelo A, Martínez-Augustin O, de Medina FS (2010) Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem 401:318–320
Medvedev AE, Kirkel AA, Kamyshanskaya NS et al (1994) Monoamine oxidase inhibition by novel antidepressant tetrindole. BiochemPharmacol 47:303–330
Gurevich IB, Kozina EA, Myagkov AA et al (2010) Automating extraction and analysis of dopaminergic axon terminals in images of frontal slices of the striatum. Patt Rec Img Anal 20:349–359
Smolen A (1990) Image analytic techniques for quantification of immunohistochemical staining in the nervous system. In: Conn M (ed) Methods in neurosciences: quantitative and qualitative microscopy. Academic Press, San Diego, pp 208–229
Borke RC, Curtis M, Ginsberg C (1993) Choline acetyltransferase and calcitonin gene-related peptide immunoreactivity in motoneurons after different types of nerve injury. J Neurocytol 22:141–153
Lucas LR, Harlan RE (1995) Cholinergic regulation of tachykinin- and enkephalin-gene expression in the rat striatum. Brain Res Mol Brain Res 30:181–195
Taranukhin AG, Taranukhina EY, Saransaari P et al (2008) Taurine reduces caspase-8 and caspase-9 expression induced by ischemia in the mouse hypothalamic nuclei. Amino Acids 34:169–174
Chang HM, Wu UI, Lan CT (2009) Melatonin preserves longevity protein (sirtuin 1) expression in the hippocampus of total sleep-deprived rats. J Pineal Res 47:211–220
Abramova MA, Calas A, Ugrumov MV (2011) Vasopressinergic neurons of the supraoptic nucleus in perinatal rats: reaction to osmotic stimulation and its regulation. Brain Struct Funct 215:195–207
Jackson-Lewis V, Jakowec M, Burke R, Przedborski S (1995) Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration 4:7–269
Hösli E, Hösli L (1997) Autoradiographic studies on the uptake of 3 H-dopamine by neurons and astrocytes in explant and primary cultures of rat CNS: effects of uptake inhibitors. Int J Dev Neurosci 15:45–53
Karakaya S, Kipp M, Beyer C (2007) Oestrogen regulates the expression and function of dopamine transporters in astrocytes of the nigrostriatal system. J Neuroendocrinol 19:682–690
Shih JC, Chen K, Ridd MJ (1999) Monoamine oxidase: from genes to behavior. Annu Rev Neurosci 22:197
Alter SP, Lenzi G, Bernstein AI, Miller GW (2013) Vesicular integrity in Parkinson’s disease. Curr Neurol Neurosci Rep 13:1–11
Morfini G, Pigino G, Opalach K et al (2007) 1-Methyl-4-phenylpyridinium affects fast axonal transport by activation of caspase and protein kinase C. Proc Natl Acad Sci USA 104:2442–2447
Chu Y, Morfini GA, Langhamer LB et al (2012) Alterations in axonal transport motor proteins in sporadic and experimental Parkinson’s disease. Brain 13:2058–2073
Cartelli D, Casagrande F, Busceti CL et al (2013) Microtubule alterations occur early in experimental parkinsonism and the microtubule stabilizer epothilone D is neuroprotective. Sci Rep 3:1837
Perez XA, Parameswaran N, Huang LZ et al (2008) Pre-synaptic dopaminergic compensation after moderate nigrostriatal damage in non-human primates. J Neurochem 105:1861–1872
Melamed E, Youdim MBH, Rosenthal J et al (1985) In vivo effect of MPTP on monoamine oxidase activity in mouse striatum. Brain Res 359:360–363
Kirchhoff J, Mørk A, Brennum LT, Sager TN (2009) Striatal extracellular dopamine levels and behavioural reversal in MPTP-lesioned mice. Neuroreport 20:482–486
Okamura N, Villemagne VL, Drago J et al (2010) In vivo measurement of vesicular monoamine transporter type 2 density in Parkinson disease with 18F-AV-133. J Nucl Med 51:223–228
Pifl C, Rajput A, Reither H et al (2014) Is Parkinson’s disease a vesicular dopamine storage disorder? Evidence from a study in isolated synaptic vesicles of human and nonhuman primate striatum. J Neurosci 34:8210–8218
De Deurwaerdère P, Di Giovanni G (2016) Serotonergic modulation of the activity of mesencephalic dopaminergic systems: therapeutic implications. Prog Neurobiol. doi:10.1016/j.pneurobio.2016.03.004
Castaneda E, Whishaw IQ, Robinson TE (1990) Changes in striatal dopamine neurotransmission assessed with microdialysis following recovery from a bilateral 6-OHDA lesion: variation as a function of lesion size. J Neurosci 106:1847–1854
Bjelke B, Strömberg I, O’Connor WT et al (1994) Evidence for volume transmission in the dopamine denervated neostriatum of the rat after a unilateral nigral 6-OHDA microinjection. Studies with systemic D-amphetamine treatment. Brain Res 662:11–24
Itzhak Y, Martin JL, Black MD, Ali SF (1999) Effect of the dopaminergic neurotoxin MPTP on cocaine-induced locomotor sensitization. Pharmacol Biochem Behav 63:101–107
Lohr KM, Miller GW (2014) VMAT2 and Parkinson’s disease: harnessing the dopamine vesicle. Expert Rev Neurother 14:1115–1117
Rozas G, Liste I, Guerra MJ, Labandeira-Garcia JL (1998) Sprouting of the serotonergic afferents into striatum after selective lesion of the dopaminergic system by MPTP in adult mice. NeurosciLett 245:151–154
Hirsch EC, Périer C, Orieux G et al (2000) Metabolic effects of nigrostriatal denervation in basal ganglia. Trends Neurosci 23:78–85
Picconi B, Piccoli G, Calabresi P (2012) Synaptic dysfunction in Parkinson’s disease. AdvExp Med Biol 970:553–572
Acknowledgements
We thank Dr. L. E. Eiden (Section on Molecular Neuroscience, Laboratory of Cellular and Molecular Regulation, NIMH, Bethesda, USA) for the kind gift of antibodies to VMAT2 and Professor N. F. Myasoedov (Institute of Molecular Genetics of the Russian Academy of Science, Moscow, Russia) for radiolabeling of DA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the experimental procedures were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996 and the UK Animals (Scientific Procedures) Act 1986 and associated guidelines or the European Communities Council Directive of 24 November 1986 (86/609/EEC) for care and use of laboratory animals and were approved by the Animal Care and Use Committee of the Institute of Developmental Biology of the Russian Academy of Sciences.
Funding
This study was supported by grant from the Federal Targeted Program Research and Development in Priority Areas of Scientific-Technological Complex of Russia for 2014–2020 years of the Ministry of Education and Science of RF (Grant: 14.604.21.0073).
Competing Interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Mingazov, E.R., Khakimova, G.R., Kozina, E.A. et al. MPTP Mouse Model of Preclinical and Clinical Parkinson’s Disease as an Instrument for Translational Medicine. Mol Neurobiol 55, 2991–3006 (2018). https://doi.org/10.1007/s12035-017-0559-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0559-6