Abstract
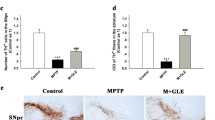
Insufficient production of nerve growth factor (NGF) is implicated in Parkinson’s disease (PD). We recently discovered that caffeic acid derivative N-propargyl caffeamide (PACA) not only potentiated NGF-induced neurite outgrowth but also attenuated 6-hydroxydopamine neurotoxicity in neuronal culture. The aim of the present study was to investigate whether PACA could increase NGF levels against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) neurotoxicity in a mouse PD model. We induced parkinsonism in mice by intraperitoneal injection of MPTP for seven consecutive days. Animal motor functions were assessed by rotarod test and pole test. Our results showed that PACA ameliorated motor impairments in MPTP-challenged mice. Based on Western blot analysis and/or immunofluorescence staining of NGF and tyrosine hydroxylase (TH), PACA preserved TH levels in the midbrain substantia nigra pars compacta. PACA also increased NGF expression while it decreased proNGF accumulation. Interestingly, NGF was widely induced in the midbrains including astrocytes. To elucidate the mechanisms by which PACA induces NGF, we focused on the effects of PACA on two neurotrophic signaling pathways, the PI3K and MEK pathways. We found that PACA induced the phosphorylation of Akt, ERK, and CREB against MPTP-mediated alterations. Importantly, PACA increased NGF levels and subsequently induced TrkA activation in MPTP-treated mice. Consistently, PACA also increased NGF levels in dopaminergic PC12 cells and primary rat midbrain neurons against N-methyl-4-phenylpyridinium iodide (MPP+) toxicity. ERK and PI3K inhibitors attenuated the effects of PACA on NGF levels. Collectively, our results suggest that PACA may rescue NGF insufficiency via sequential activation of PI3K/Akt, ERK1/2, and CREB signaling pathways.

ᅟ






Similar content being viewed by others
Abbreviations
- PACA:
-
N-propargyl caffeamide
- NGF:
-
nerve growth factor
- PD:
-
Parkinson’s disease
- TH:
-
Tyrosine hydroxylase
- MPTP:
-
1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine
- 6-OHDA:
-
6-hydroxydopamine
- SNpc:
-
Substantia nigra pars compacta
- TrkA:
-
Tropomyosin receptor kinase A
- PI3K:
-
Phosphoinositide 3-kinase
- ERK:
-
Extracellular Signal-regulated Kinase-1
- MPP+ :
-
N-methyl-4-phenylpyridinium iodide
References
Brown CA, Cheng EM, Hays RD, Vassar SD, Vickrey BG (2009) SF-36 includes less Parkinson disease (PD)-targeted content but is more responsive to change than two PD-targeted health-related quality of life measures. Qual Life Res 18(9):1219–1237. doi:10.1007/s11136-009-9530-y
Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M et al (2003) D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest 112(6):892–901. doi:10.1172/JCI18797
Jonnala RR, Buccafusco JJ (2001) Inhibition of nerve growth factor signaling by peroxynitrite. J Neurosci Res 63(1):27–34. doi:10.1002/1097-4547(20010101)63:1<27::AID-JNR4>3.0.CO;2-#
Bruno MA, Cuello AC (2012) Cortical peroxynitration of nerve growth factor in aged and cognitively impaired rats. Neurobiol Aging 33(9):1927–1937. doi:10.1016/j.neurobiolaging.2011.09.031
Tillerson JL, Caudle WM, Reveron ME, Miller GW (2003) Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience 119(3):899–911. doi:10.1016/S0306-4522(03)00096-4
Le W, Sayana P, Jankovic J (2014) Animal models of Parkinson’s disease: a gateway to therapeutics? Neurotherapeutics 11(1):92–110. doi:10.1007/s13311-013-0234-1
Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S, Ichinose H, Nagatsu T (1999) Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci Lett 270(1):45–48. doi:10.1016/S0304-3940(99)00463-2
van Dijk KD, Teunissen CE, Drukarch B, Jimenez CR, Groenewegen HJ, Berendse HW, van de Berg WD (2010) Diagnostic cerebrospinal fluid biomarkers for Parkinson’s disease: a pathogenetically based approach. Neurobiol Dis 39(3):229–241. doi:10.1016/j.nbd.2010.04.020
Aron L, Klein R (2011) Repairing the parkinsonian brain with neurotrophic factors. Trends Neurosci 34(2):88–100. doi:10.1016/j.tins.2010.11.001
Aloe L, Rocco ML, Bianchi P, Manni L (2012) Nerve growth factor: from the early discoveries to the potential clinical use. J Transl Med 10. doi:10.1186/1479-5876-10-239
Olson L, Backman L, Ebendal T, Eriksdotterjonhagen M, Hoffer B, Humpel C, Freedman R, Giacobini M et al (1994) Role of growth-factors in degeneration and regeneration in the central-nervous-system—clinical-experiences with NGF in Parkinson’s and Alzheimer’s diseases. J Neurol 242(1):S12–S15. doi:10.1007/BF00939233
Wu DD, Huang L, Zhang L, Wu LY, Li YC, Feng LY (2012) LLDT-67 attenuates MPTP-induced neurotoxicity in mice by up-regulating NGF expression. Acta Pharmacol Sin 33(9):1187–1194. doi:10.1038/aps. 2012.88
Kaplan DR, Martinzanca D, Parada LF (1991) Tyrosine phosphorylation and tyrosine kinase-activity of the Trk Protooncogene product induced by NGF. Nature 350(6314):158–160. doi:10.1038/350158a0
Chung J, Kubota H, Ozaki Y, Uda S, Kuroda S (2010) Timing-dependent actions of NGF required for cell differentiation. PLoS One 5(2):e9011. doi:10.1371/journal.pone.0009011
Bonuccelli U, Del Dotto P (2006) New pharmacologic horizons in the treatment of Parkinson’s disease. Neurology 67(7 Suppl 2):S30–S38. doi:10.1212/WNL.67.7_suppl_2.S30
Muller T, Woitalla D, Hauptmann B, Fowler B, Kuhn W (2001) Decrease of methionine and S-adenosylmethionine and increase of homocysteine in treated patients with Parkinson’s disease. Neurosci Lett 308(1):54–56. doi:10.1016/S0304-3940(01)01972-3
Troster AI (2011) A precis of recent advances in the neuropsychology of mild cognitive impairment(s) in Parkinson’s disease and a proposal of preliminary research criteria. J Int Neuropsychol Soc 17(3):393–406. doi:10.1017/S1355617711000257
Chao MV, Rajagopal R, Lee FS (2006) Neurotrophin signalling in health and disease. Clin Sci 110(2):167–173. doi:10.1042/CS20050163
Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK (2013) GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Therapeut 138(2):155–175. doi:10.1016/j.pharmthera.2013.01.004
Yang C, Zhao J, Cheng Y, Le XC, Rong J (2015) N-propargyl caffeate amide (PACA) potentiates nerve growth factor (NGF)-induced neurite outgrowth and attenuates 6-hydroxydopamine (6-OHDA)-induced toxicity by activating the Nrf2/HO-1 pathway. ACS Chem Neurosci 6(9):1560–1569. doi:10.1021/acschemneuro.5b00115
Zhao J, Luo D, Liang Z, Lao L, Rong J (2016) Plant natural product Puerarin ameliorates depressive behaviors and chronic pain. Mol Neurobiol. doi:10.1007/s12035-016-9870-x
Fiuza SM, Gomes C, Teixeira LJ, da Cruz MTG, Cordeiro MNDS, Milhazes N, Borges F, Marques MPM (2004) Phenolic acid derivatives with potential anticancer properties—a structure-activity relationship study. Part 1: methyl, propyl and octyl esters of caffeic and gallic acids. Bioorgan Med Chem 12(13):3581–3589. doi:10.1016/j.bmc.2004.04.026
Bindoli A, Rigobello MP (2013) Principles in redox signaling: from chemistry to functional significance. Antioxid Redox Sign 18(13):1557–1593. doi:10.1089/ars.2012.4655
Zhao J, Cheng YY, Fan W, Yang CB, Ye SF, Cui W, Wei W, Lao LX et al (2015) Botanical drug Puerarin coordinates with nerve growth factor in the regulation of neuronal survival and neuritogenesis via activating ERK1/2 and PI3K/Akt signaling pathways in the neurite extension process. Cns Neurosci Ther 21(1):61–70. doi:10.1111/cns.12334
Liu J, Huang D, Xu J, Tong J, Wang Z, Huang L, Yang Y, Bai X et al (2015) Tiagabine protects dopaminergic neurons against neurotoxins by inhibiting microglial activation. Sci Rep 5:15720. doi:10.1038/srep15720
Cheng YY, Xia ZY, Han YF, Rong JH (2016) Plant natural product formononetin protects rat cardiomyocyte H9c2 cells against oxygen glucose deprivation and reoxygenation via inhibiting ROS formation and promoting GSK-3 beta phosphorylation. Oxidative Med Cell Longev. doi:10.1155/2016/2060874
Sanders LH, McCoy J, Hu X, Mastroberardino PG, Dickinson BC, Chang CJ, Chu CT, Van Houten B et al (2014) Mitochondrial DNA damage: molecular marker of vulnerable nigral neurons in Parkinson’s disease. Neurobiol Dis 70:214–223. doi:10.1016/j.nbd.2014.06.014
Nie SK, Xu Y, Chen GG, Ma K, Han C, Guo ZL, Zhang ZT, Ye KG et al (2015) Small molecule TrkB agonist deoxygedunin protects nigrostriatal dopaminergic neurons from 6-OHDA and MPTP induced neurotoxicity in rodents. Neuropharmacology 99:448–458. doi:10.1016/j.neuropharm.2015.08.016
Longo FM, Massa SM (2013) Small-molecule modulation of neurotrophin receptors: a strategy for the treatment of neurological disease. Nat Rev Drug Discov 12(7):507–525. doi:10.1038/nrd4024
Friedman WJ, Greene LA (1999) Neurotrophin signaling via Trks and p75. Exp Cell Res 253(1):131–142. doi:10.1006/excr.1999.4705
Hennigan A, O’Callaghan RM, Kelly AM (2007) Neurotrophins and their receptors: roles in plasticity, neurodegeneration and neuroprotection. Biochem Soc T 35:424–427. doi:10.1042/BST0350424
Huang EJ, Reichardt LF (2003) Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72:609–642. doi:10.1146/annurev.biochem.72.121801.161629
Deshmukh M, Johnson EM (1997) Programmed cell death in neurons: focus on the pathway of nerve growth factor deprivation-induced death of sympathetic neurons. Mol Pharmacol 51(6):897–906. doi:10.1124/mol.51.6.897
Takadera T, Ohyashiki T (1998) Apoptotic cell death and CPP32-like activation induced by thapsigargin and their prevention by nerve growth factor in PC12 cells. BBA-Mol Cell Res 1401(1):63–71. doi:10.1016/S0167-4889(97)00116-X
Andsberg G, Kokaia Z, Bjorklund A, Lindvall O, Martinez-Serrano A (1998) Amelioration of ischaemia-induced neuronal death in the rat striatum by NGF-secreting neural stem cells. Eur J Neurosci 10(6):2026–2036. doi:10.1046/j.1460-9568.1998.00214.x
Barcelona PF, Saragovi HU (2015) A pro-nerve growth factor (proNGF) and NGF binding protein, alpha(2)-macroglobulin, differentially regulates p75 and TrkA receptors and is relevant to neurodegeneration ex vivo and in vivo. Mol Cell Biol 35(19):3396–3408. doi:10.1128/MCB.00544-15
Pedre LL, Fuentes NP, Gonzalez LA, McRae A, Sanchez TS, Lescano LB, Gonzalez RM (2002) Nerve growth factor levels in Parkinson’s disease and experimental parkinsonian rats. Brain Res 952(1):122–127. doi:10.1016/S0006-8993(02)03222-5
Halvorsen EM, Dennis J, Keeney P, Sturgill TW, Tuttle JB, Bennett JB Jr (2002) Methylpyridinium (MPP(+))- and nerve growth factor-induced changes in pro- and anti-apoptotic signaling pathways in SH-SY5Y neuroblastoma cells. Brain Res 952(1):98–110. doi:10.1016/S0006-8993(02)03216-X
Salinas M, Diaz R, Abraham NG, Ruiz de Galarreta CM, Cuadrado A (2003) Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J Biol Chem 278(16):13898–13904. doi:10.1074/jbc.M209164200
Vaudry D, Stork PJ, Lazarovici P, Eiden LE (2002) Signaling pathways for PC12 cell differentiation: making the right connections. Science 296(5573):1648–1649. doi:10.1126/science.1071552
Wang T, Liu YY, Wang X, Yang N, Zhu HB, Zuo PP (2010) Protective effects of octacosanol on 6-hydroxydopamine-induced parkinsonism in rats via regulation of ProNGF and NGF signaling. Acta Pharmacol Sin 31(7):765–774. doi:10.1038/aps.2010.69
Sanchez MG, Ruiz-Llorente L, Sanchez AM, Diaz-Laviada I (2003) Activation of phosphoinositide 3-kinase/PKB pathway by CB1 and CB2 cannabinoid receptors expressed in prostate PC-3 cells. Involvement in Raf-1 stimulation and NGF induction. Cell Signal 15(9):851–859. doi:10.1016/S0898-6568(03)00036-6
Venkatesan R, Ji E, Kim SY (2015) Phytochemicals that regulate neurodegenerative disease by targeting neurotrophins: a comprehensive review. Biomed Res Int. doi:10.1155/2015/814068
Jurič DM, Šuput D, Brvar M (2016) Hyperbaric oxygen preserves neurotrophic activity of carbon monoxide-exposed astrocytes. Toxicol Lett 253:1–6. doi:10.1016/j.toxlet.2016.04.019
Zhang H, Xiao J, Hu Z, Xie M, Wang W, He D (2016) Blocking transient receptor potential vanilloid 2 channel in astrocytes enhances astrocyte-mediated neuroprotection after oxygen–glucose deprivation and reoxygenation. Eur J Neurosci 44(7):2493–2503. doi:10.1111/ejn.13352
El Omri A, Han J, Kawada K, Ben Abdrabbah M, Isoda H (2012) Luteolin enhances cholinergic activities in PC12 cells through ERK1/2 and PI3K/Akt pathways. Brain Res 1437:16–25. doi:10.1016/j.brainres.2011.12.019
Zhou J, Ping FF, Lv WT, Feng JY, Shang J (2014) Interleukin-18 directly protects cortical neurons by activating PI3K/AKT/NF-kappa B/CREB pathways. Cytokine 69(1):29–38. doi:10.1016/j.cyto.2014.05.003
Finkbeiner S (2000) CREB couples neurotrophin signals to survival messages. Neuron 25(1):11–14. doi:10.1016/S0896-6273(00)80866-1
Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME (1997) CREB: a major mediator of neuronal neurotrophin responses. Neuron 19(5):1031–1047. doi:10.1016/S0896-6273(00)80395-5
Acknowledgements
This work was supported by General Research Fund (GRF) grants (775812M and 17120915) from the Research Grants Council of Hong Kong and the Seed Funding for Basic Research Program from University of Hong Kong.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interest.
Rights and permissions
About this article
Cite this article
Luo, D., Zhao, J., Cheng, Y. et al. N-Propargyl Caffeamide (PACA) Ameliorates Dopaminergic Neuronal Loss and Motor Dysfunctions in MPTP Mouse Model of Parkinson’s Disease and in MPP+-Induced Neurons via Promoting the Conversion of proNGF to NGF. Mol Neurobiol 55, 2258–2267 (2018). https://doi.org/10.1007/s12035-017-0486-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0486-6