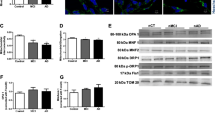
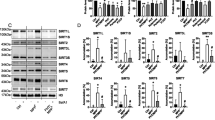
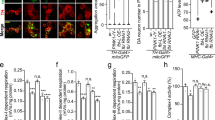
Abstract
Alterations in microtubule-dependent transport, mitochondrial dysfunction, and autophagic pathology are involved in neurodegeneration observed in sporadic Parkinson’s disease. However, the mechanistic link connecting these events remains elusive. We observed that NAD+ metabolism is altered in sporadic Parkinson’s disease patient-derived cells, which contributes to Sirtuin-2 activation and subsequent decrease in acetylated-α-tubulin levels. Pharmacological inhibition of sirtuin-2 deacetylase activity selectively enhanced α-tubulin acetylation and facilitated the trafficking and clearance of misfolded proteins. Sirtuin-2 knock-out mice neurons had no alteration in microtubule assembly after exposure to MPP+, allowing the maintenance of a normal autophagic flux. These data were validated using MPTP-treated sirtuin-2 knock-out mice, where no alterations in motor behavior were observed. Biochemical analysis of sporadic Parkinson’s disease patient brains supports the in vitro and in vivo data. Our data provide strong evidence that sirtuin-2 controls the functional ability of the autophagic system through acetylation and highlight the association between mitochondrial metabolism and neurodegeneration in sporadic Parkinson’s disease.







Similar content being viewed by others
Abbreviations
- PD:
-
Parkinson’s disease
- SNpc:
-
Substantia nigra pars compacta
- LBs:
-
Lewy Bodies
- MT:
-
Microtubule
- SNCA:
-
α-synuclein
- SIRT2:
-
Sirtuin-2
- HDAC6:
-
Histone deacetylase 6
- MTOC:
-
Microtubule-organizing center)
- MPP+ :
-
1-methyl-4-phenylpyridium
- MPTP:
-
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- TH:
-
Tyrosine hydroxylase
- 6OHDA:
-
6-hydroxydopamine
References
Forno LS (1996) Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol 55:259–272
Lee HJ, Khoshaghideh F, Lee S, Lee SJ (2006) Impairment of microtubule-dependent trafficking by overexpression of alpha-synuclein. Eur J Neurosci 24:3153–3162
Esteves AR, Arduino DM, Swerdlow RH, Oliveira CR, Cardoso SM (2009) Oxidative stress involvement in alpha-synuclein oligomerization in Parkinson’s disease cybrids. Antioxid Redox Signal 11:439–448
Esteves AR, Arduino DM, Swerdlow RH, Oliveira CR, Cardoso SM (2010) Microtubule depolymerization potentiates alpha-synuclein oligomerization. Front Aging Neurosci 1:5
Arduino DM, Esteves AR, Cortes L, Silva DF, Patel B, Grazina M, Swerdlow RH, Oliveira CR et al (2012) Mitochondrial metabolism in Parkinson’s disease impairs quality control autophagy by hampering microtubule-dependent traffic. Hum Mol Genet 21:4680–4702
Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM et al (2007) Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science 317:516–519
North BJ, Marshall BL, Borra MT, Denu JM, Verdin E (2003) The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell 11:437–444
Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF et al (2002) HDAC6 is a microtubule-associated deacetylase. Nature 417:455–458
Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL (2003) Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci U S A 100:4389–4394
Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ (2006) Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol 16:2166–2172
Xie R, Nguyen S, McKeehan WL, Liu L (2010) Acetylated microtubules are required for fusion of autophagosomes with lysosomes. BMC Cell Biol 11:89
Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, McKeith I, Quinn N, Sethi KD et al (2003) Movement disorders society scientific issues committee report: SIC task force appraisal of clinical diagnostic criteria for parkinsonian disorders. Mov Disord 18:467–486
Esteves AR, Domingues AF, Ferreira IL, Januario C, Swerdlow RH, Oliveira CR, Cardoso SM (2008) Mitochondrial function in Parkinson’s disease cybrids containing an nt2 neuron-like nuclear background. Mitochondrion 8:219–228
Sodja C, Fang H, Dasgupta T, Ribecco M, Walker PR, Sikorska M (2002) Identification of functional dopamine receptors in human teratocarcinoma NT2 cells. Brain Res Mol Brain Res 99:83–91
Serrano L, Martinez-Redondo P, Marazuela-Duque A, Vazquez BN, Dooley SJ, Voigt P, Beck DB, Kane-Goldsmith N et al (2013) The tumor suppressor SirT2 regulates cell cycle progression and genome stability by modulating the mitotic deposition of H4K20 methylation. Genes Dev 27:639–653
Gao HM, Liu B, Zhang W, Hong JS (2003) Synergistic dopaminergic neurotoxicity of MPTP and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson’s disease. FASEB J 17:1957–1959
Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M (2001) The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem 76:1265–1274
Monville C, Torres EM, Dunnett SB (2006) Comparison of incremental and accelerating protocols of the rotarod test for the assessment of motor deficits in the 6-OHDA model. J Neurosci Methods 158:219–223
Pereira FC, Gough B, Macedo TR, Ribeiro CF, Ali SF, Binienda ZK (2011) Buprenorphine modulates methamphetamine-induced dopamine dynamics in the rat caudate nucleus. Neurotox Res 19:94–101
Silva DF, Selfridge JE, Lu JEL, Roy N, Hutfles L, Burns JM, Michaelis EK, Yan S et al (2013) Bioenergetic flux, mitochondrial mass and mitochondrial morphology dynamics in AD and MCI cybrid cell lines. Hum Mol Genet 22:3931–3946
Esteves AR, M GF, Santos D, Januario C, Cardoso SM (2014) The upshot of LRRK2 inhibition to Parkinson’s disease paradigm. Mol Neurobiol
Scaduto RC Jr, Grotyohann LW (1999) Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J 76:469–477
Esteves AR, Lu J, Rodova M, Onyango I, Lezi E, Dubinsky R, Lyons KE, Pahwa R et al (2010) Mitochondrial respiration and respiration-associated proteins in cell lines created through Parkinson’s subject mitochondrial transfer. J Neurochem 113:674–682
Pollak N, Dolle C, Ziegler M (2007) The power to reduce: pyridine nucleotides—small molecules with a multitude of functions. Biochem J 402:205–218
Vemuri GN, Eiteman MA, McEwen JE, Olsson L, Nielsen J (2007) Increasing NADH oxidation reduces overflow metabolism in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 104:2402–2407
Vinogradov AD (2008) NADH/NAD+ interaction with NADH: ubiquinone oxidoreductase (complex I). Biochim Biophys Acta 1777:729–734
Orsomando G, Cialabrini L, Amici A, Mazzola F, Ruggieri S, Conforti L, Janeckova L, Coleman MP et al (2012) Simultaneous single-sample determination of NMNAT isozyme activities in mouse tissues. PLoS One 7:e53271
Lawson M, Uciechowska U, Schemies J, Rumpf T, Jung M, Sippl W (2010) Inhibitors to understand molecular mechanisms of NAD(+)-dependent deacetylases (sirtuins). Biochim Biophys Acta 1799:726–739
Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP (2003) The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 115:727–738
Pandey UB, Nie Z, Batlevi Y, McCray BA, Ritson GP, Nedelsky NB, Schwartz SL, DiProspero NA et al (2007) HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 447:859–863
Kochl R, Hu XW, Chan EY, Tooze SA (2006) Microtubules facilitate autophagosome formation and fusion of autophagosomes with endosomes. Traffic 7:129–145
Rubinsztein DC, Cuervo AM, Ravikumar B, Sarkar S, Korolchuk V, Kaushik S, Klionsky DJ (2009) In search of an "autophagomometer". Autophagy 5:585–589
Alim MA, Ma QL, Takeda K, Aizawa T, Matsubara M, Nakamura M, Asada A, Saito T et al (2004) Demonstration of a role for alpha-synuclein as a functional microtubule-associated protein. J Alzheimers Dis 6:435–442 discussion 443-439
Langston JW, Ballard P, Tetrud JW, Irwin I (1983) Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219:979–980
Henchcliffe C, Beal MF (2008) Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 4:600–609
Cardoso SM (2011) The mitochondrial cascade hypothesis for Parkinson’s disease. Curr Pharm Des 17:3390–3397
Santos D, Esteves AR, Silva DF, Januario C, Cardoso SM (2014) The impact of mitochondrial fusion and fission modulation in sporadic Parkinson’s disease. Mol Neurobiol
Magni G, Orsomando G, Raffelli N, Ruggieri S (2008) Enzymology of mammalian NAD metabolism in health and disease. Front Biosci 13:6135–6154
White AT, Schenk S (2012) NAD(+)/NADH and skeletal muscle mitochondrial adaptations to exercise. Am J Physiol Endocrinol Metab 303:E308–E321
Arduino DM, Esteves AR, Oliveira CR, Cardoso SM (2010) Mitochondrial metabolism modulation: a new therapeutic approach for Parkinson’s disease. CNS Neurol Disord Drug Targets 9:105–119
Janke C, Bulinski JC (2011) Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol 12:773–786
Skoge RH, Dolle C, Ziegler M (2014) Regulation of SIRT2-dependent alpha-tubulin deacetylation by cellular NAD levels. DNA Repair (Amst) 23:33–38
Suzuki K, Koike T (2007) Mammalian Sir2-related protein (SIRT) 2-mediated modulation of resistance to axonal degeneration in slow Wallerian degeneration mice: a crucial role of tubulin deacetylation. Neuroscience 147:599–612
Godena VK, Brookes-Hocking N, Moller A, Shaw G, Oswald M, Sancho RM, Miller CC, Whitworth AJ et al (2014) Increasing microtubule acetylation rescues axonal transport and locomotor deficits caused by LRRK2 Roc-COR domain mutations. Nat Commun 5:5245
Lee JY, Koga H, Kawaguchi Y, Tang W, Wong E, Gao YS, Pandey UB, Kaushik S et al (2010) HDAC6 controls autophagosome maturation essential for ubiquitin-selective quality-control autophagy. EMBO J 29:969–980
Liu L, Arun A, Ellis L, Peritore C, Donmez G (2014) SIRT2 enhances 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced nigrostriatal damage via apoptotic pathway. Front Aging Neurosci 6:184
Burke RE, O’Malley K (2013) Axon degeneration in Parkinson’s disease. Exp Neurol 246:72–83
Patel VP, Chu CT (2014) Decreased SIRT2 activity leads to altered microtubule dynamics in oxidatively-stressed neuronal cells: implications for Parkinson’s disease. Exp Neurol 257:170–181
Cartelli D, Casagrande F, Busceti CL, Bucci D, Molinaro G, Traficante A, Passarella D, Giavini E et al (2013) Microtubule alterations occur early in experimental parkinsonism and the microtubule stabilizer epothilone D is neuroprotective. Sci Rep 3:1837
Chen X, Wales P, Quinti L, Zuo F, Moniot S, Herisson F, Rauf NA, Wang H et al (2015) The sirtuin-2 inhibitor AK7 is neuroprotective in models of Parkinson’s disease but not amyotrophic lateral sclerosis and cerebral ischemia. PLoS One 10:e0116919
Guan Q, Wang M, Chen H, Yang L, Yan Z, Wang X (2016) Aging-related 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurochemial and behavioral deficits and redox dysfunction: improvement by AK-7. Exp Gerontol 82:19–29
Wang X, Guan Q, Wang M, Yang L, Bai J, Yan Z, Zhang Y, Liu Z (2015) Aging-related rotenone-induced neurochemical and behavioral deficits: role of SIRT2 and redox imbalance, and neuroprotection by AK-7. Drug Des Devel Ther 9:2553–2563
Silva DF, Esteves AR, Oliveira CR, Cardoso SM (2016) Mitochondrial metabolism power SIRT2-dependent deficient traffic causing Alzheimer’s-disease related pathology. Mol Neurobiol.
Acknowledgements
We are grateful to Dr. Russell H Swerdlow for providing the PD patients samples. Human brain samples were obtained in the Neurological Tissue Bank, Biobanc-Hospital Clinic-IDIBAPS and from a generous gift from Professor I Ferrer Abizanda, Bellvitge Hospital Universitari, Institut Català de la Salut, Barcelona, Spain.
Author Contributions
SMC designed the experiments; ARE executed the experiments; DMA and DFS performed the autophagy-related experiments in cybrid cell lines; SDV and FCP carried out the in vivo experiments, namely, injecting MPTP to mice and determining dopamine levels; SMC, ARE, and FCP carried out the data analysis; and SMC and ARE wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by funds from PTDC/SAU-NEU/102710/2008, PEst-C/SAU/LA0001/2011–2013, Janssen Neuroscience Award, from Janssen Pharmaceutics to SM Cardoso. AR Esteves and DF Silva are supported by Post-Doctoral Fellowships from Portuguese Foundation for Science and Technology (FCT-MCTES, Portugal).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Supplementary Figure 1
(DOCX 2428 kb)
Supplementary Figure 2
(DOCX 5234 kb)
Supplementary Figure 3
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Esteves, A.R., Arduíno, D.M., Silva, D.F. et al. Mitochondrial Metabolism Regulates Microtubule Acetylome and Autophagy Trough Sirtuin-2: Impact for Parkinson’s Disease. Mol Neurobiol 55, 1440–1462 (2018). https://doi.org/10.1007/s12035-017-0420-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0420-y