Abstract
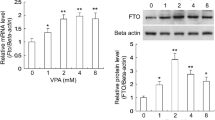
Upregulation of sodium channel SCN3A expression in epileptic tissues is known to contribute to enhancing neuronal excitability and the development of epilepsy. Therefore, certain strategies to reduce SCN3A expression may be helpful for seizure control. Here, we reveal a novel role of valproate (VPA) in the epigenetic downregulation of Scn3a expression. We found that VPA, instead of carbamazepine (CBZ) and lamotrigine (LTG), could significantly downregulate Scn3a expression in mouse Neuro-2a cells. Luciferase assays and CpG methylation analyses showed that VPA induced the methylation at the -39C site in Scn3a promoter which decreased the promoter activity. We further showed that VPA downregulated the expression of methyl-CpG-binding domain protein 2 (MBD2) at the posttranscriptional level and knockdown of MBD2 increased Scn3a expression. In addition, we found that VPA induced the expression of fat mass and obesity-associated (FTO) protein and FTO knockdown abolished the repressive effects of VPA on MBD2 and Nav1.3 expressions. Furthermore, VPA, instead of other two anticonvulsant drugs, induced the expressions of Scn3a and Mbd2 and reduced Fto expression in the hippocampus of VPA-treated seizure mice. Taken together, this study suggests an epigenetic pathway for the VPA-induced downregulation of Scn3a expression, which provides a possible role of this pathway in the anticonvulsant action of VPA.






Similar content being viewed by others
References
Fisher RS, van Emde BW, Blume W, Elger C, Genton P, Lee P, Engel J Jr (2005) Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 46:470–472
Klein JP, Khera DS, Nersesyan H, Kimchi EY, Waxman SG, Blumenfeld H (2004) Dysregulation of sodium channel expression in cortical neurons in a rodent model of absence epilepsy. Brain Res 1000:102–109
Aronica E, Gorter JA (2007) Gene expression profile in temporal lobe epilepsy. Neuroscientist 13:100–108
Altar CA, Laeng P, Jurata LW, Brockman JA, Lemire A, Bullard J, Bukhman YV, Young TA et al (2004) Electroconvulsive seizures regulate gene expression of distinct neurotrophic signaling pathways. J Neurosci 24:2667–2677
Beckh S, Noda M, Lubbert H, Numa S (1989) Differential regulation of three sodium channel messenger RNAs in the rat central nervous system during development. The EMBO J 8:3611–3616
Brysch W, Creutzfeldt OD, Luno K, Schlingensiepen R, Schlingensiepen KH (1991) Regional and temporal expression of sodium channel messenger RNAs in the rat brain during development. Exp Brain Res 86:562–567
Whitaker WR, Faull RL, Waldvogel HJ, Plumpton CJ, Emson PC, Clare JJ (2001) Comparative distribution of voltage-gated sodium channel proteins in human brain. Brain Res 88:37–53
Guo F, Yu N, Cai JQ, Quinn T, Zong ZH, Zeng YJ, Hao LY (2008) Voltage-gated sodium channel Nav1.1, Nav1.3 and beta1 subunit were up-regulated in the hippocampus of spontaneously epileptic rat. Brain Res Bull 75:179–187
Yu S, Li S, Shu H, Zhang C, He J, Fan X, Yang H (2012) Upregulated expression of voltage-gated sodium channel Nav1.3 in cortical lesions of patients with focal cortical dysplasia type IIb. Neuroreport 23:407–411
Chen C, Westenbroek RE, Xu X, Edwards CA, Sorenson DR, Chen Y, McEwen DP, O’Malley HA et al (2004) Mice lacking sodium channel beta1 subunits display defects in neuronal excitability, sodium channel expression, and nodal architecture. J Neurosci 24:4030–4042
Li HJ, Wan RP, Tang LJ, Liu SJ, Zhao QH, Gao MM, Yi YH, Liao WP et al (2015) Alteration of Scn3a expression is mediated via CpG methylation and MBD2 in mouse hippocampus during postnatal development and seizure condition. Biochimica et Biophysica Acta 1849:1–9
Cummins TR, Aglieco F, Renganathan M, Herzog RI, Dib-Hajj SD, Waxman SG (2001) Nav1.3 sodium channels: rapid repriming and slow closed-state inactivation display quantitative differences after expression in a mammalian cell line and in spinal sensory neurons. J Neurosci 21:5952–5961
Calleja S, Salas-Puig J, Ribacoba R, Lahoz CH (2001) Evolution of juvenile myoclonic epilepsy treated from the outset with sodium valproate. Seizure 10:424–427
Coppola G, Auricchio G, Federico R, Carotenuto M, Pascotto A (2004) Lamotrigine versus valproic acid as first-line monotherapy in newly diagnosed typical absence seizures: an open-label, randomized, parallel-group study. Epilepsia 45:1049–1053
Dean JC, Penry JK (1988) Valproate monotherapy in 30 patients with partial seizures. Epilepsia 29:140–144
Erenberg G, Rothner AD, Henry CE (1960) Cruse RP (1982) Valproic acid in the treatment of intractable absence seizures in children: a single-blind clinical and quantitative EEG study. Am J Dis Child 136:526–529
Gurvich N, Klein PS (2002) Lithium and valproic acid: parallels and contrasts in diverse signaling contexts. Pharmacol Ther 96:45–66
Toth M (2005) The epsilon theory: a novel synthesis of the underlying molecular and electrophysiological mechanisms of primary generalized epilepsy and the possible mechanism of action of valproate. Med hypotheses 64:267–272
Johannessen CU (2000) Mechanisms of action of valproate: a commentatory. Neurochem Int 37:103–110
Loscher W (1999) Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol 58:31–59
Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F et al (2001) Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J 20:6969–6978
Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS (2001) Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem 276:36734–36741
Milutinovic S, D’Alessio AC, Detich N, Szyf M (2007) Valproate induces widespread epigenetic reprogramming which involves demethylation of specific genes. Carcinogenesis 28:560–571
Cervoni N, Szyf M (2001) Demethylase activity is directed by histone acetylation. J Biol Chem 276:40778–40787
Cervoni N, Detich N, Seo SB, Chakravarti D, Szyf M (2002) The oncoprotein Set/TAF-1beta, an inhibitor of histone acetyltransferase, inhibits active demethylation of DNA, integrating DNA methylation and transcriptional silencing. J Biol Chem 277:25026–25031
Asghari V, Wang JF, Reiach JS, Young LT (1998) Differential effects of mood stabilizers on Fos/Jun proteins and AP-1 DNA binding activity in human neuroblastoma SH-SY5Y cells. Brain Res 58:95–102
Chen G, Yuan PX, Jiang YM, Huang LD, Manji HK (1999) Valproate robustly enhances AP-1 mediated gene expression. Brain Res 64:52–58
Fukuchi M, Nii T, Ishimaru N, Minamino A, Hara D, Takasaki I, Tabuchi A, Tsuda M (2009) Valproic acid induces up- or down-regulation of gene expression responsible for the neuronal excitation and inhibition in rat cortical neurons through its epigenetic actions. Neurosci Res 65:35–43
Petty SJ, Kantor S, Lawrence KM, Berkovic SF, Collins M, Hill KD, Makovey J, Sambrook PN et al (2014) Weight and fat distribution in patients taking valproate: a valproate-discordant gender-matched twin and sibling pair study. Epilepsia 55:1551–1557
Rauchenzauner M, Griesmacher A, Tatarczyk T, Haberlandt E, Strasak A, Zimmerhackl LB, Falkensammer G, Luef G et al (2010) Chronic antiepileptic monotherapy, bone metabolism, and body composition in non-institutionalized children. Dev Med Child Neurol 52:283–288
Zhang L, Chu X, Wang H, Xie H, Guo C, Cao L, Zhou X, Wang G et al (2013) Dysregulations of UDP-glucuronosyltransferases in rats with valproic acid and high fat diet induced fatty liver. Eur J Pharmacol 721:277–285
Church C, Moir L, McMurray F, Girard C, Banks GT, Teboul L, Wells S, Bruning JC et al (2010) Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet 42:1086–1092
Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Bruning JC, Ruther U (2009) Inactivation of the Fto gene protects from obesity. Nature 458:894–898
Miller-Delaney SF, Das S, Sano T, Jimenez-Mateos EM, Bryan K, Buckley PG, Stallings RL, Henshall DC (2012) Differential DNA methylation patterns define status epilepticus and epileptic tolerance. J Neurosci 32:1577–1588
Smemo S, Tena JJ, Kim KH, Gamazon ER, Sakabe NJ, Gomez-Marin C, Aneas I, Credidio FL et al (2014) Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature 507:371–375
Yang J, Loos RJ, Powell JE, Medland SE, Speliotes EK, Chasman DI, Rose LM, Thorleifsson G et al (2012) FTO genotype is associated with phenotypic variability of body mass index. Nature 490:267–272
Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS, Yeo GS, McDonough MA et al (2007) The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 318:1469–1472
Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T et al (2012) N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nature Chem Biol 7:885–887
Rowles J, Wong M, Powers R, Olsen M (2012) FTO, RNA epigenetics and epilepsy. Epigenetics 7:1094–1097
Jaenisch R, Bird A (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33(Suppl):245–254
Gos M (2013) Epigenetic mechanisms of gene expression regulation in neurological diseases. Acta Neurobiol Exp 73:19–37
Jakovcevski M, Akbarian S (2012) Epigenetic mechanisms in neurological disease. Nat Med 18:1194–1204
Urdinguio RG, Sanchez-Mut JV, Esteller M (2009) Epigenetic mechanisms in neurological diseases: genes, syndromes, and therapies. Lancet Neurol 8:1056–1072
Kobow K, Jeske I, Hildebrandt M, Hauke J, Hahnen E, Buslei R, Buchfelder M, Weigel D et al (2009) Increased reelin promoter methylation is associated with granule cell dispersion in human temporal lobe epilepsy. J Neuropathol Exp Neurol 68:356–364
Heinrich C, Nitta N, Flubacher A, Muller M, Fahrner A, Kirsch M, Freiman T, Suzuki F et al (2006) Reelin deficiency and displacement of mature neurons, but not neurogenesis, underlie the formation of granule cell dispersion in the epileptic hippocampus. J Neurosci 26:4701–4713
Kundakovic M, Chen Y, Costa E, Grayson DR (2007) DNA methyltransferase inhibitors coordinately induce expression of the human reelin and glutamic acid decarboxylase 67 genes. Mol Pharmacol 71:644–653
Balasubramanian D, Deng AX, Doudney K, Hampton MB, Kennedy MA (2015) Valproic acid exposure leads to upregulation and increased promoter histone acetylation of sepiapterin reductase in a serotonergic cell line. Neuropharmacology 99:79–88
Deng GF, Qin JM, Sun XS, Kuang ZY, Su T, Zhao QH, Shi YW, Liu XR et al (2011) Promoter analysis of mouse Scn3a gene and regulation of the promoter activity by GC box and CpG methylation. J Mol Neurosci 44:115–121
Michaelis M, Kohler N, Reinisch A, Eikel D, Gravemann U, Doerr HW, Nau H, Cinatl J Jr (2004) Increased human cytomegalovirus replication in fibroblasts after treatment with therapeutical plasma concentrations of valproic acid. Biochem Pharmacol 68:531–538
Loscher W (2002) Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS drugs 16:669–694
Perucca E (2002) Pharmacological and therapeutic properties of valproate: a summary after 35 years of clinical experience. CNS drugs 16:695–714
McLean MJ, Macdonald RL (1986) Sodium valproate, but not ethosuximide, produces use- and voltage-dependent limitation of high frequency repetitive firing of action potentials of mouse central neurons in cell culture. J Pharmacol Exp Ther 237:1001–1011
Owens MJ, Nemeroff CB (2003) Pharmacology of valproate. Psychopharmacol Bull 37(Suppl 2):17–24
Dong E, Chen Y, Gavin DP, Grayson DR, Guidotti A (2010) Valproate induces DNA demethylation in nuclear extracts from adult mouse brain. Epigenetics 5:730–735
Gobbi G, Janiri L (2006) Sodium- and magnesium-valproate in vivo modulate glutamatergic and GABAergic synapses in the medial prefrontal cortex. Psychopharmacology 185:255–262
Qureshi IA, Mehler MF (2010) Epigenetic mechanisms underlying human epileptic disorders and the process of epileptogenesis. Neurobiol Dis 39:53–60
Szyf M (2009) Epigenetics, DNA methylation, and chromatin modifying drugs. Annu Rev Pharmacol Toxicol 49:243–263
Mattson RH, Cramer JA, Collins JF (1992) A comparison of valproate with carbamazepine for the treatment of complex partial seizures and secondarily generalized tonic-clonic seizures in adults. The Department of Veterans Affairs Epilepsy Cooperative Study No. 264 Group. N Engl J Med 327:765–771
Gilliam F, Vazquez B, Sackellares JC, Chang GY, Messenheimer J, Nyberg J, Risner ME, Rudd GD (1998) An active-control trial of lamotrigine monotherapy for partial seizures. Neurology 51:1018–1025
Wheless JW, Clarke DF, Carpenter D (2005) Treatment of pediatric epilepsy: expert opinion, 2005. J Child Neurol 20(Suppl 1):S1–56; quiz S59-60
Zdrojowy-Welna A, Tupikowska M, Kolackov K, Bednarek-Tupikowska G (2014) The role of fat mass and obesity-associated gene (FTO) in obesity—an overview. Endokrynol Pol 65:224–231
Liu G, Yarov-Yarovoy V, Nobbs M, Clare JJ, Scheuer T, Catterall WA (2003) Differential interactions of lamotrigine and related drugs with transmembrane segment IVS6 of voltage-gated sodium channels. Neuropharmacology 44:413–422
Cummings C, Stewart M, Stevenson M, Morrow J, Nelson J (2011) Neurodevelopment of children exposed in utero to lamotrigine, sodium valproate and carbamazepine. Arch Dis Child 96:643–647
Roopra A, Dingledine R, Hsieh J (2012) Epigenetics and epilepsy. Epilepsia 53(Suppl 9):2–10
Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR (2012) Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149:1635–1646
Acknowledgments
We thank Yan Luo for helpful discussion and technical assistance. This work was supported by the National Natural Science Foundation of China (grant numbers 81371436, 31070928, 81171073, and 81471149), and partially supported by the Collaborative Innovation Center for Neurogenetics and Channelopathies. We are grateful to the He Shanheng Charity Foundation for contributing to the development of this institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The mouse studies were approved by ethical committee for the use of experimental animals of Guangzhou Medical University.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Na-Na Tan and Hui-Ling Tang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tan, NN., Tang, HL., Lin, GW. et al. Epigenetic Downregulation of Scn3a Expression by Valproate: a Possible Role in Its Anticonvulsant Activity. Mol Neurobiol 54, 2831–2842 (2017). https://doi.org/10.1007/s12035-016-9871-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9871-9