Abstract
Cerebellar damage and granular and Purkinje cell loss in sporadic Creutzfeldt–Jakob disease (sCJD) highlight a critical involvement of the cerebellum during symptomatic progression of the disease. In this project, global proteomic alterations in the cerebellum of brain from the two most prevalent subtypes (MM1 and VV2) of sCJD were studied. Two-dimensional gel electrophoresis (2DE) coupled mass spectrometric identification revealed 40 proteins in MM1 and 43 proteins in VV2 subtype to be differentially expressed. Of those, 12 proteins showed common differential expression in their expression between two subtypes. Differentially expressed proteins mainly belonged to (i) cell cycle, gene expression and cell death; (ii) cellular stress response/oxidative stress (OS) and (iii) signal transduction and synaptic functions, related molecular functions. We verified 10 differentially expressed proteins at transcriptional and translational level as well. Interestingly, protein deglycase DJ-1 (an antioxidative protein) showed an increase in its messenger RNA (mRNA) expression in both MM1 and VV2 subtypes but protein expression only in VV2 subtype in cerebellum of sCJD patients. Nuclear translocalization of DJ-1 confirmed its expressional alteration due to OS in sCJD. Downstream experiments showed the activation of nuclear factor erythroid-2 related factor 2 (Nrf2)/antioxidative response element (ARE) pathway. DJ-1 protein concentration was significantly increased during the clinical phase in cerebrospinal fluid of sCJD patients and also at presymptomatic and symptomatic stages in cerebellum of humanized PrP transgenic mice inoculated with sCJD (MM1 and VV2) brain. These results suggest the implication of oxidative stress during the pathophysiology of sCJD.







Similar content being viewed by others
Abbreviations
- sCJD:
-
Sporadic Creutzfeldt–Jakob disease
- PrPC :
-
Cellular prion protein
- PrPSc :
-
Scrapie form of prion proteins
- 2DE:
-
Two-dimensional gel electrophoresis
- OS:
-
Oxidative stress
- CSF:
-
Cerebrospinal fluid
References
Prusiner SB, DeArmond SJ (1994) Prion diseases and neurodegeneration. Annu Rev Neurosci 17:311–339
Gambetti P, Kong Q, Zou W, Parchi P, Chen SG (2003) Sporadic and familial CJD: classification and characterisation. Br Med Bull 66:213–239
Puoti G, Bizzi A, Forloni G, Safar JG, Tagliavini F, Gambetti P (2012) Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol 11(7):618–628
Gawinecka J, Cardone F, Asif AR, De PA, Wemheuer WM, Schulz-Schaeffer WJ, Pocchiari M, Zerr I (2012) Sporadic creutzfeldt-Jakob disease subtype-specific alterations of the brain proteome: impact on Rab3a recycling. Proteomics 12(23–24):3610–3620
Parchi P, Giese A, Capellari S, Brown P, Schulz-Schaeffer W, Windl O, Zerr I, Budka H et al (1999) Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 46(2):224–233
Yang Q, Hashizume Y, Yoshida M, Wang Y (1999) Neuropathological study of cerebellar degeneration in prion disease. Neuropathology 19(1):33–39
KH E, Chen SH, Ho MH, Desmond JE (2014) A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum Brain Mapp 35(2):593–615
Kim SG, Ugurbil K, Strick PL (1994) Activation of a cerebellar output nucleus during cognitive processing. Science 265(5174):949–951
Zhang J, Keene CD, Pan C, Montine KS, Montine TJ (2008) Proteomics of human neurodegenerative diseases. J Neuropathol Exp Neurol 67(10):923–932
Yun SW, Gerlach M, Riederer P, Klein MA (2006) Oxidative stress in the brain at early preclinical stages of mouse scrapie. Exp Neurol 201(1):90–98
Harish G, Venkateshappa C, Mahadevan A, Pruthi N, Bharath MM, Shankar SK (2011) Effect of storage time, postmortem interval, agonal state, and gender on the postmortem preservation of glial fibrillary acidic protein and oxidatively damaged proteins in human brains. Biopreserv Biobank 9(4):379–387
Llorens F, Ansoleaga B, Garcia-Esparcia P, Zafar S, Grau-Rivera O, Lopez-Gonzalez I, Blanco R, Carmona M et al (2013) PrP mRNA and protein expression in brain and PrP(c) in CSF in Creutzfeldt-Jakob disease MM1 and VV2. Prion 7(5):383–393
Llorens F, Lopez-Gonzalez I, Thune K, Carmona M, Zafar S, Andreoletti O, Zerr I, Ferrer I (2014) Subtype and regional-specific neuroinflammation in sporadic creutzfeldt-jakob disease. Front Aging Neurosci 6:198
Zafar S, Asif AR, Ramljak S, Tahir W, Schmitz M, Zerr I (2014) Anchorless 23-230 PrPC interactomics for elucidation of PrPC protective role. Mol Neurobiol 49(3):1385–1399
Ramljak S, Asif AR, Armstrong VW, Wrede A, Groschup MH, Buschmann A, Schulz-Schaeffer W, Bodemer W et al (2008) Physiological role of the cellular prion protein (PrPc): protein profiling study in two cell culture systems. J Proteome Res 7(7):2681–2695
Blum H, Beier H, Gross HJ (1987) Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8(2):93–99
Cassard H, Torres JM, Lacroux C, Douet JY, Benestad SL, Lantier F, Lugan S, Lantier I et al (2014) Evidence for zoonotic potential of ovine scrapie prions. Nat Commun 5:5821
Padilla D, Beringue V, Espinosa JC, Andreoletti O, Jaumain E, Reine F, Herzog L, Gutierrez-Adan A et al (2011) Sheep and goat BSE propagate more efficiently than cattle BSE in human PrP transgenic mice. PLoS Pathog 7(3):e1001319
Zafar S, Schmitz M, Younus N, Tahir W, Shafiq M, Llorens F, Ferrer I, Andeoletti O et al (2015) Creutzfeldt-Jakob disease subtype-specific regional and temporal regulation of ADP Ribosylation factor-1-dependent rho/MLC pathway at pre-clinical stage. J Mol Neurosci 56(2):329–348
Zafar S, Younas N, Correia S, Shafiq M, Tahir W, Schmitz M, Ferrer I, Andreoletti O et al (2016) Strain-specific altered regulatory response of Rab7a and Tau in Creutzfeldt-Jakob disease and Alzheimer’s disease. Mol Neurobiol. doi:10.1007/s12035-016-9694-8
Kruse N, Schulz-Schaeffer WJ, Schlossmacher MG, Mollenhauer B (2012) Development of electrochemiluminescence-based singleplex and multiplex assays for the quantification of alpha-synuclein and other proteins in cerebrospinal fluid. Methods 56(4):514–518
Blackinton J, Lakshminarasimhan M, Thomas KJ, Ahmad R, Greggio E, Raza AS, Cookson MR, Wilson MA (2009) Formation of a stabilized cysteine sulfinic acid is critical for the mitochondrial function of the parkinsonism protein DJ-1. J Biol Chem 284(10):6476–6485
Kim SJ, Park YJ, Hwang IY, Youdim MB, Park KS, Oh YJ (2012) Nuclear translocation of DJ-1 during oxidative stress-induced neuronal cell death. Free Radic Biol Med 53(4):936–950
Liu C, Chen Y, Kochevar IE, Jurkunas UV (2014) Decreased DJ-1 leads to impaired Nrf2-regulated antioxidant defense and increased UV-A-induced apoptosis in corneal endothelial cells. Invest Ophthalmol Vis Sci 55(9):5551–5560
Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N et al (1997) An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236(2):313–322
Venugopal R, Jaiswal AK (1996) Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A 93(25):14960–14965
Ayala YM, De CL, Avendano-Vazquez SE, Dhir A, Romano M, D’Ambrogio A, Tollervey J, Ule J et al (2011) TDP-43 regulates its mRNA levels through a negative feedback loop. EMBO J 30(2):277–288
Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X et al (2008) Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med 178(6):592–604
Michel B, Ferguson A, Johnson T, Bender H, Meyerett-Reid C, Wyckoff AC, Pulford B, Telling GC et al (2013) Complement protein C3 exacerbates prion disease in a mouse model of chronic wasting disease. Int Immunol 25(12):697–702
Mokhtar SH, Bakhuraysah MM, Cram DS, Petratos S (2013) The beta-amyloid protein of Alzheimer’s disease: communication breakdown by modifying the neuronal cytoskeleton. Int J Alzheimers Dis 2013:910502
Zhou M, Ottenberg G, Sferrazza GF, Hubbs C, Fallahi M, Rumbaugh G, Brantley AF, Lasmezas CI (2015) Neuronal death induced by misfolded prion protein is due to NAD+ depletion and can be relieved in vitro and in vivo by NAD+ replenishment. Brain 138(Pt 4):992–1008
Zhou RH, Kokame K, Tsukamoto Y, Yutani C, Kato H, Miyata T (2001) Characterization of the human NDRG gene family: a newly identified member, NDRG4, is specifically expressed in brain and heart. Genomics 73(1):86–97
Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K et al (1998) Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17(9):2596–2606
Buratti E, Baralle FE (2010) The multiple roles of TDP-43 in pre-mRNA processing and gene expression regulation. RNA Biol 7(4):420–429
Buratti E, Baralle M, Baralle FE (2013) From single splicing events to thousands: the ambiguous step forward in splicing research. Brief Funct Genomics 12(1):3–12
Buratti E, Romano M, Baralle FE (2013) TDP-43 high throughput screening analyses in neurodegeneration: advantages and pitfalls. Mol Cell Neurosci 56:465–474
Iguchi Y, Katsuno M, Niwa J, Takagi S, Ishigaki S, Ikenaka K, Kawai K, Watanabe H et al (2013) Loss of TDP-43 causes age-dependent progressive motor neuron degeneration. Brain 136(Pt 5):1371–1382
Licatalosi DD, Darnell RB (2010) RNA processing and its regulation: global insights into biological networks. Nat Rev Genet 11(1):75–87
Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B et al (2009) Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323(5918):1208–1211
Perrone-Bizzozero N, Bolognani F (2002) Role of HuD and other RNA-binding proteins in neural development and plasticity. J Neurosci Res 68(2):121–126
Bekenstein U, Soreq H (2013) Heterogeneous nuclear ribonucleoprotein A1 in health and neurodegenerative disease: from structural insights to post-transcriptional regulatory roles. Mol Cell Neurosci 56:436–446
Berson A, Barbash S, Shaltiel G, Goll Y, Hanin G, Greenberg DS, Ketzef M, Bcker AJ et al (2012) Cholinergic-associated loss of hnRNP-A/B in Alzheimer’s disease impairs cortical splicing and cognitive function in mice. EMBO Mol Med 4(8):730–742
Park SJ, Lee H, Jo DS, Jo YK, Shin JH, Kim HB, Seo HM, Rubinsztein DC et al (2015) Heterogeneous nuclear ribonucleoprotein A1 post-transcriptionally regulates Drp1 expression in neuroblastoma cells. Biochim Biophys Acta 1849(12):1423–1431
Amadio M, Scapagnini G, Davinelli S, Calabrese V, Govoni S, Pascale A (2014) Involvement of ELAV RNA-binding proteins in the post-transcriptional regulation of HO-1. Front Cell Neurosci 8:459
Becker M, Kuhse J, Kirsch J (2013) Effects of two elongation factor 1A isoforms on the formation of gephyrin clusters at inhibitory synapses in hippocampal neurons. Histochem Cell Biol 140(6):603–609
Boese AS, Saba R, Campbell K, Majer A, Medina S, Burton L, Booth TF, Chong P et al (2016) MicroRNA abundance is altered in synaptoneurosomes during prion disease. Mol Cell Neurosci 71:13–24
Saba R, Goodman CD, Huzarewich RL, Robertson C, Booth SA (2008) A miRNA signature of prion induced neurodegeneration. PLoS One 3(11):e3652
Tsuchiya N, Ochiai M, Nakashima K, Ubagai T, Sugimura T, Nakagama H (2007) SND1, a component of RNA-induced silencing complex, is up-regulated in human colon cancers and implicated in early stage colon carcinogenesis. Cancer Res 67(19):9568–9576
Tahara EB, Barros MH, Oliveira GA, Netto LE, Kowaltowski AJ (2007) Dihydrolipoyl dehydrogenase as a source of reactive oxygen species inhibited by caloric restriction and involved in Saccharomyces cerevisiae aging. FASEB J 21(1):274–283
Gibson GE, Park LC, Sheu KF, Blass JP, Calingasan NY (2000) The alpha-ketoglutarate dehydrogenase complex in neurodegeneration. Neurochem Int 36(2):97–112
Sullivan PG, Brown MR (2005) Mitochondrial aging and dysfunction in Alzheimer’s disease. Prog Neuro-Psychopharmacol Biol Psychiatry 29(3):407–410
Brown AM, Gordon D, Lee H, Caudy M, Haroutunian V, Blass JP (2004) Substantial linkage disequilibrium across the dihydrolipoyl succinyltransferase gene region without Alzheimer’s disease association. Neurochem Res 29(3):629–635
Brown AM, Gordon D, Lee H, Wavrant-De VF, Cellini E, Bagnoli S, Nacmias B, Sorbi S et al (2007) Testing for linkage and association across the dihydrolipoyl dehydrogenase gene region with Alzheimer’s disease in three sample populations. Neurochem Res 32(4–5):857–869
Tumani H, Shen G, Peter JB, Bruck W (1999) Glutamine synthetase in cerebrospinal fluid, serum, and brain: a diagnostic marker for Alzheimer disease? Arch Neurol 56(10):1241–1246
Plaitakis A, Shashidharan P (2000) Glutamate transport and metabolism in dopaminergic neurons of substantia nigra: implications for the pathogenesis of Parkinson’s disease. J Neurol 247(Suppl 2):II25–II35
Zhang P, Hu X, Xu X, Chen Y, Bache RJ (2011) Dimethylarginine dimethylaminohydrolase 1 modulates endothelial cell growth through nitric oxide and Akt. Arterioscler Thromb Vasc Biol 31(4):890–897
Butterfield DA, Gnjec A, Poon HF, Castegna A, Pierce WM, Klein JB, Martins RN (2006) Redox proteomics identification of oxidatively modified brain proteins in inherited Alzheimer’s disease: an initial assessment. J Alzheimers Dis 10(4):391–397
Gawinecka J, Dieks J, Asif AR, Carimalo J, Heinemann U, Streich JH, Dihazi H, Schulz-Schaeffer W et al (2010) Codon 129 polymorphism specific cerebrospinal fluid proteome pattern in sporadic Creutzfeldt-Jakob disease and the implication of glycolytic enzymes in prion-induced pathology. J Proteome Res 9(11):5646–5657
Mulder C, Wahlund LO, Blomberg M, de JS, van Kamp GJ, Scheltens P, Teerlink T (2002) Alzheimer’s disease is not associated with altered concentrations of the nitric oxide synthase inhibitor asymmetric dimethylarginine in cerebrospinal fluid. J Neural Transm (Vienna) 109(9):1203–1208
Molenaar RJ, Radivoyevitch T, Maciejewski JP, van Noorden CJ, Bleeker FE (2014) The driver and passenger effects of isocitrate dehydrogenase 1 and 2 mutations in oncogenesis and survival prolongation. Biochim Biophys Acta 1846(2):326–341
Yamamoto H, Kokame K, Okuda T, Nakajo Y, Yanamoto H, Miyata T (2011) NDRG4 protein-deficient mice exhibit spatial learning deficits and vulnerabilities to cerebral ischemia. J Biol Chem 286(29):26158–26165
Vidal AE, Boiteux S, Hickson ID, Radicella JP (2001) XRCC1 coordinates the initial and late stages of DNA abasic site repair through protein-protein interactions. EMBO J 20(22):6530–6539
Gencer M, Dasdemir S, Cakmakoglu B, Cetinkaya Y, Varlibas F, Tireli H, Kucukali CI, Ozkok E et al (2012) DNA repair genes in Parkinson’s disease. Genet Test Mol Biomarkers 16(6):504–507
Diedrich M, Kitada T, Nebrich G, Koppelstaetter A, Shen J, Zabel C, Klose J, Mao L (2011) Brain region specific mitophagy capacity could contribute to selective neuronal vulnerability in Parkinson’s disease. Proteome Sci 9:59
Smeyne M, Smeyne RJ (2013) Glutathione metabolism and Parkinson’s disease. Free Radic Biol Med 62:13–25
Kabil O, Banerjee R (2012) Characterization of patient mutations in human persulfide dioxygenase (ETHE1) involved in H2S catabolism. J Biol Chem 287(53):44561–44567
Pettinati I, Brem J, McDonough MA, Schofield CJ (2015) Crystal structure of human persulfide dioxygenase: structural basis of ethylmalonic encephalopathy. Hum Mol Genet 24(9):2458–2469
Di DF, Sultana R, Tiu GF, Scheff NN, Perluigi M, Cini C, Butterfield DA (2010) Protein levels of heat shock proteins 27, 32, 60, 70, 90 and thioredoxin-1 in amnestic mild cognitive impairment: an investigation on the role of cellular stress response in the progression of Alzheimer disease. Brain Res 1333:72–81
Power JH, Asad S, Chataway TK, Chegini F, Manavis J, Temlett JA, Jensen PH, Blumbergs PC et al (2008) Peroxiredoxin 6 in human brain: molecular forms, cellular distribution and association with Alzheimer’s disease pathology. Acta Neuropathol 115(6):611–622
Silva-Adaya D, Gonsebatt ME, Guevara J (2014) Thioredoxin system regulation in the central nervous system: experimental models and clinical evidence. Oxidative Med Cell Longev 2014:590808
Baulac S, Lu H, Strahle J, Yang T, Goldberg MS, Shen J, Schlossmacher MG, Lemere CA et al (2009) Increased DJ-1 expression under oxidative stress and in Alzheimer’s disease brains. Mol Neurodegener 4:12
Klein MA, Kaeser PS, Schwarz P, Weyd H, Xenarios I, Zinkernagel RM, Carroll MC, Verbeek JS et al (2001) Complement facilitates early prion pathogenesis. Nat Med 7(4):488–492
Harischandra DS, Kondru N, Martin DP, Kanthasamy A, Jin H, Anantharam V, Kanthasamy AG (2014) Role of proteolytic activation of protein kinase Cdelta in the pathogenesis of prion disease. Prion 8(1):143–153
Eaton CJ, Cabrera IE, Servin JA, Wright SJ, Cox MP, Borkovich KA (2012) The guanine nucleotide exchange factor RIC8 regulates conidial germination through Galpha proteins in Neurospora crassa. PLoS One 7(10):e48026
Rizo J, Sudhof TC (2002) Snares and Munc18 in synaptic vesicle fusion. Nat Rev Neurosci 3(8):641–653
Triplett JC, Zhang Z, Sultana R, Cai J, Klein JB, Bueler H, Butterfield DA (2015) Quantitative expression proteomics and phosphoproteomics profile of brain from PINK1 knockout mice: insights into mechanisms of familial Parkinson’s disease. J Neurochem 133(5):750–765
Hsuan J, Cockcroft S 2001 The PITP family of phosphatidylinositol transfer proteins. Genome Biol 2(9):REVIEWS3011
Betteridge DJ (2000) What is oxidative stress? Metabolism 49(2 Suppl 1):3–8
Dringen R, Gutterer JM, Hirrlinger J (2000) Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem 267(16):4912–4916
Gandhi S, Abramov AY (2012) Mechanism of oxidative stress in neurodegeneration. Oxidative Med Cell Longev 2012:428010
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Salganik RI (2001) The benefits and hazards of antioxidants: controlling apoptosis and other protective mechanisms in cancer patients and the human population. J Am Coll Nutr 20(5 Suppl):464S–472S
Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, Reaume AG, Scott RW et al (1998) Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science 281(5384):1851–1854
Valentine JS, Hart PJ (2003) Misfolded CuZnSOD and amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 100(7):3617–3622
Freixes M, Rodriguez A, Dalfo E, Ferrer I (2006) Oxidation, glycoxidation, lipoxidation, nitration, and responses to oxidative stress in the cerebral cortex in creutzfeldt-Jakob disease. Neurobiol Aging 27(12):1807–1815
Pamplona R, Naudi A, Gavin R, Pastrana MA, Sajnani G, Ilieva EV, Del Rio JA, Portero-Otin M et al (2008) Increased oxidation, glycoxidation, and lipoxidation of brain proteins in prion disease. Free Radic Biol Med 45(8):1159–1166
Bai J, Nakamura H, Hattori I, Tanito M, Yodoi J (2002) Thioredoxin suppresses 1-methyl-4-phenylpyridinium-induced neurotoxicity in rat PC12 cells. Neurosci Lett 321(1–2):81–84
Kahle PJ, Waak J, Gasser T (2009) DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders. Free Radic Biol Med 47(10):1354–1361
Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D et al (2004) The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci U S A 101(24):9103–9108
Junn E, Jang WH, Zhao X, Jeong BS, Mouradian MM (2009) Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J Neurosci Res 87(1):123–129
Kato I, Maita H, Takahashi-Niki K, Saito Y, Noguchi N, Iguchi-Ariga SM, Ariga H (2013) Oxidized DJ-1 inhibits p53 by sequestering p53 from promoters in a DNA-binding affinity-dependent manner. Mol Cell Biol 33(2):340–359
Chanas SA, Jiang Q, McMahon M, McWalter GK, McLellan LI, Elcombe CR, Henderson CJ, Wolf CR et al (2002) Loss of the Nrf2 transcription factor causes a marked reduction in constitutive and inducible expression of the glutathione S-transferase Gsta1, Gsta2, Gstm1, Gstm2, Gstm3 and Gstm4 genes in the livers of male and female mice. Biochem J 365(Pt 2):405–416
Takagi Y, Hattori I, Nozaki K, Mitsui A, Ishikawa M, Hashimoto N, Yodoi J (2000) Excitotoxic hippocampal injury is attenuated in thioredoxin transgenic mice. J Cereb Blood Flow Metab 20(5):829–833
Zhou F, Gomi M, Fujimoto M, Hayase M, Marumo T, Masutani H, Yodoi J, Hashimoto N et al (2009) Attenuation of neuronal degeneration in thioredoxin-1 overexpressing mice after mild focal ischemia. Brain Res 1272:62–70
Im JY, Lee KW, Junn E, Mouradian MM (2010) DJ-1 protects against oxidative damage by regulating the thioredoxin/ASK1 complex. Neurosci Res 67(3):203–208
Pompella A, Visvikis A, Paolicchi A, De TV, Casini AF (2003) The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol 66(8):1499–1503
Zhou W, Freed CR (2005) DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J Biol Chem 280(52):43150–43158
Li Y, Yan M, Yang J, Raman I, Du Y, Min S, Fang X, Mohan C et al (2014) Glutathione S-transferase mu 2-transduced mesenchymal stem cells ameliorated anti-glomerular basement membrane antibody-induced glomerulonephritis by inhibiting oxidation and inflammation. Stem Cell Res Ther 5(1):19
Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP (2006) DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci U S A 103(41):15091–15096
Cheng X, Chapple SJ, Patel B, Puszyk W, Sugden D, Yin X, Mayr M, Siow RC et al (2013) Gestational diabetes mellitus impairs Nrf2-mediated adaptive antioxidant defenses and redox signaling in fetal endothelial cells in utero. Diabetes 62(12):4088–4097
Moinova HR, Mulcahy RT (1999) Up-regulation of the human gamma-glutamylcysteine synthetase regulatory subunit gene involves binding of Nrf-2 to an electrophile responsive element. Biochem Biophys Res Commun 261(3):661–668
Sekhar KR, Crooks PA, Sonar VN, Friedman DB, Chan JY, Meredith MJ, Starnes JH, Kelton KR et al (2003) NADPH oxidase activity is essential for Keap1/Nrf2-mediated induction of GCLC in response to 2-indol-3-yl-methylenequinuclidin-3-ols. Cancer Res 63(17):5636–5645
Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S (2002) Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res 62(18):5196–5203
Acknowledgements
This work was supported by a grant from Helmholtz-Alberta Initiative-Infectious Diseases Research (HAI-IDR) and APRI-Human prions—distinguishing sporadic from familial forms via structure and function as well as from the DZNE clinical project (Helmholtz). The study was performed within the recently established Clinical Dementia Center at the University Medical Hospital Göttingen and was partly supported by grants from the EU Joint Program Neurodegenerative Disease Research (JPND-DEMTEST) (Biomarker based diagnosis of rapid progressive dementias-optimization of diagnostic protocols, 01ED1201A). We also pay our special thanks to Dr. André Fischer (DZNE, Goettingen) and Dr. Stefan Bonn (DZNE, Goettingen) for sharing their lab facilities, Dr. Christine Stadelmann-Nessler (UMG, Goettingen) for providing hemeoxygenase 1 primary antibody and Dr. Hassan Dihazi (UMG, Goettingen) for peroxiredoxin 6 primary antibody.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(SF3 33,161 kb)
ESM 2
(XLS 140 kb)
ESM 3
(DOCX 94 kb)
ESM 4
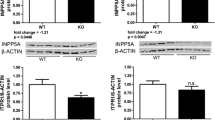
Supplementary Fig. 1: Four silver stained 2DE gels corresponding to four samples in each biological group (Control, MM1 and VV2).Supplementary Fig. 2: Characterization of samples and methodology used for proteomics: (A) Western blot analysis for total PrP protein expression in cerebellum from MM1 (n = 4) and VV2 (n = 4) subtypes of sCJD along with their age-matched NDCs (n = 4) by using anti-PrP (SAF70) antibody is shown. Densitometeric analysis showed an increase of total PrP levels both in MM1 and VV2 subtypes as already reported before. GAPDH was used as a loading control. (B) Analysis for proteinase K (PK) resistant pathogenic PrPres from cerebellum in MM1 and VV2 subtypes of sCJD was performed by PK digestion of samples from MM1 (n = 4) and VV2 (n = 4) subtypes of sCJD along with age-matched NDCs (n = 4) from cerebellum of brain with 2.5 μg/ml of PK followed by separation on SDS-PAGE gel. Western blotting was performed by using anti-PrP (SAF70) antibody. Densitometeric analysis showed the presence of PK resistant PrP: PrPres levels both in MM1 and VV2 subtypes as compared to age-matched NDCs as already reported before. “E” is for empty well which was kept intentionally empty to avoid an overflow of samples between two adjacent wells. (C) SOD1 was identified by MS/ MS in protein spot 801 whose spot intensity was significantly increased in MM1 subtype. SOD1 is a protein which is already reported to be increased in MM1 and VV2 subtypes of sCJD. In this study, qPCR results showed a significant increase in mRNA expression of SOD1 in both MM1 (n = 15) and VV2 (n = 10) as compared with age matched NDC (n = 15) samples. Western blot results showed a significant increase in protein expression of SOD1 in both MM1 (n = 4) and VV2 (n = 4) as compared with age matched NDC (n = 4) samples. Statistical significance was calculated with one way ANOVA followed by Turkey post test to compare all pairs of columns. *p < 0.05, **p < 0.005, ***p < 0.001. These results of total PrP expression, PKres PrP expression and SOD1 expressional alteration in both MM1 and VV2 subtypes indicated the well characterization of samples, method of quantification of protein spots on 2DE gels with Delta2D DECODON software and MS/MS method of protein identification in this study.Supplementary Fig. 3: Regulation of spots used in validation of proteomics findings after mass-spectrometry.Supplementary Table 1: Primary Antibodies usedSupplementary Table 2: Secondary Antibodies usedSupplementary Table 3: Oligonucleotides (probes) used foqPCRsSupplementary Table 4: Kits and commercial buffers used (DOCX 605 kb)
Rights and permissions
About this article
Cite this article
Tahir, W., Zafar, S., Llorens, F. et al. Molecular Alterations in the Cerebellum of Sporadic Creutzfeldt–Jakob Disease Subtypes with DJ-1 as a Key Regulator of Oxidative Stress. Mol Neurobiol 55, 517–537 (2018). https://doi.org/10.1007/s12035-016-0294-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0294-4