Abstract
Spinal cord injuries (SCIs) are devastating conditions of the central nervous system (CNS) for which there are no restorative therapies. Neuronal death at the primary lesion site and in remote regions that are functionally connected to it is one of the major contributors to neurological deficits following SCI.
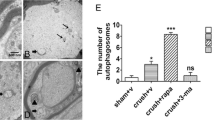
Disruption of autophagic flux induces neuronal death in many CNS injuries, but its mechanism and relationship with remote cell death after SCI are unknown. We examined the function and effects of the modulation of autophagy on the fate of axotomized rubrospinal neurons in a rat model of spinal cord dorsal hemisection (SCH) at the cervical level. Following SCH, we observed an accumulation of LC3-positive autophagosomes (APs) in the axotomized neurons 1 and 5 days after injury. Furthermore, this accumulation was not attributed to greater initiation of autophagy but was caused by a decrease in AP clearance, as demonstrated by the build-up of p62, a widely used marker of the induction of autophagy. In axotomized rubrospinal neurons, the disruption of autophagic flux correlated strongly with remote neuronal death and worse functional recovery. Inhibition of AP biogenesis by 3-methyladenine (3-MA) significantly attenuated remote degeneration and improved spontaneous functional recovery, consistent with the detrimental effects of autophagy in remote damage after SCH. Collectively, our results demonstrate that autophagic flux is blocked in axotomized neurons on SCI and that the inhibition of AP formation improves their survival. Thus, autophagy is a promising target for the development of therapeutic interventions in the treatment of SCIs.







Similar content being viewed by others
Abbreviations
- CNS:
-
Central nervous system
- SCI:
-
Spinal cord injury
- SCH:
-
Spinal cord hemisection
- 3-MA:
-
3-Methyladenine;
- Rapa:
-
Rapamycin
- FB:
-
Fast-blue
- RN:
-
Red nucleus
- LC3:
-
Light chain 3
- mTOR:
-
Mammalian target of rapamycin
- APs:
-
Autophagosomes
- AMPK:
-
Adenosine monophosphate-activated protein kinase
- cyt-c:
-
Cytochrome-c
- Comp C:
-
Compound C
- i.c.v.:
-
Intracerebroventricularly
References
Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR (2004) Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J 4(4):451–464. doi:10.1016/j.spinee.2003.07.007
Cadotte DW, Fehlings MG (2011) Spinal cord injury: a systematic review of current treatment options. Clin Orthop Relat Res 469(3):732–741. doi:10.1007/s11999-010-1674-0
Wu B, Ren X (2009) Promoting axonal myelination for improving neurological recovery in spinal cord injury. J Neurotrauma 26(10):1847–1856. doi:10.1089/neu.2008.0551
Bradbury EJ, Carter LM (2011) Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury. Brain Res Bull 84(4–5):306–316. doi:10.1016/j.brainresbull.2010.06.015
Bonner JF, Steward O (2015) Repair of spinal cord injury with neuronal relays: from fetal grafts to neural stem cells. Brain Res 1619:115–123. doi:10.1016/j.brainres.2015.01.006
Ramer LM, Ramer MS, Bradbury EJ (2014) Restoring function after spinal cord injury: towards clinical translation of experimental strategies. The Lancet Neurology 13(12):1241–1256. doi:10.1016/S1474-4422(14)70144-9
Viscomi MT, Molinari M (2014) Remote neurodegeneration: multiple actors for one play. Mol Neurobiol 50(2):368–389. doi:10.1007/s12035-013-8629-x
Block F, Dihne M, Loos M (2005) Inflammation in areas of remote changes following focal brain lesion. Prog Neurobiol 75(5):342–365. doi:10.1016/j.pneurobio.2005.03.004
Klionsky DJ (2005) The molecular machinery of autophagy: unanswered questions. J Cell Sci 118(Pt 1):7–18. doi:10.1242/jcs.01620
Levine B, Klionsky DJ (2004) Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6(4):463–477
Mizushima N (2007) Autophagy: process and function. Genes Dev 21(22):2861–2873. doi:10.1101/gad.1599207
Rubinsztein DC, DiFiglia M, Heintz N, Nixon RA, Qin ZH, Ravikumar B, Stefanis L, Tolkovsky A (2005) Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy 1(1):11–22
Nixon RA (2013) The role of autophagy in neurodegenerative disease. Nat Med 19(8):983–997. doi:10.1038/nm.3232
Sarkar S (2013) Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem Soc Trans 41(5):1103–1130. doi:10.1042/BST20130134
Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM (2005) Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol 64(2):113–122
Nixon RA, Yang DS (2011) Autophagy failure in Alzheimer’s disease—locating the primary defect. Neurobiol Dis 43(1):38–45. doi:10.1016/j.nbd.2011.01.021
Rubinsztein DC (2006) The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 443(7113):780–786. doi:10.1038/nature05291
Lipinski MM, Wu J, Faden AI, Sarkar C (2015) Function and mechanisms of autophagy in brain and spinal cord trauma. Antioxid Redox Signal 23(6):565–577. doi:10.1089/ars.2015.6306
Viscomi MT, D’Amelio M, Cavallucci V, Latini L, Bisicchia E, Nazio F, Fanelli F, Maccarrone M, Moreno S, Cecconi F, Molinari M (2012) Stimulation of autophagy by rapamycin protects neurons from remote degeneration after acute focal brain damage. Autophagy 8(2):222–235. doi:10.4161/auto.8.2.18599
Cavallucci V, Bisicchia E, Cencioni MT, Ferri A, Latini L, Nobili A, Biamonte F, Nazio F, Fanelli F, Moreno S, Molinari M, Viscomi MT, D’Amelio M (2014) Acute focal brain damage alters mitochondrial dynamics and autophagy in axotomized neurons. Cell Death Dis 5:e1545. doi:10.1038/cddis.2014.511
Kanno H, Ozawa H, Sekiguchi A, Itoi E (2009) Spinal cord injury induces upregulation of Beclin 1 and promotes autophagic cell death. Neurobiol Dis 33(2):143–148. doi:10.1016/j.nbd.2008.09.009
Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Itoi E (2011) Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine 36(22):E1427–E1434. doi:10.1097/BRS.0b013e3182028c3a
Tang P, Hou H, Zhang L, Lan X, Mao Z, Liu D, He C, Du H, Zhang L (2014) Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol Neurobiol 49(1):276–287. doi:10.1007/s12035-013-8518-3
Liu S, Sarkar C, Dinizo M, Faden AI, Koh EY, Lipinski MM, Wu J (2015) Disrupted autophagy after spinal cord injury is associated with ER stress and neuronal cell death. Cell Death Dis 6:e1582. doi:10.1038/cddis.2014.527
Ribas VT, Schnepf B, Challagundla M, Koch JC, Bahr M, Lingor P (2015) Early and sustained activation of autophagy in degenerating axons after spinal cord injury. Brain Pathol 25(2):157–170. doi:10.1111/bpa.12170
Klionsky DJ, Abdalla FC, Abeliovich H, et al. (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8(4):445–544
Klionsky DJ, Abdelmohsen K, Abe A, et al. (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12(1):1–222. doi:10.1080/15548627.2015.1100356
Chan EY, Kir S, Tooze SA (2007) siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J Biol Chem 282(35):25464–25474. doi:10.1074/jbc.M703663200
Egan D, Kim J, Shaw RJ, Guan KL (2011) The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 7(6):643–644
Kim J, Kundu M, Viollet B, Guan KL (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13(2):132–141. doi:10.1038/ncb2152
Jung CH, Ro SH, Cao J, Otto NM, Kim DH (2010) mTOR regulation of autophagy. FEBS Lett 584(7):1287–1295. doi:10.1016/j.febslet.2010.01.017
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132(1):27–42. doi:10.1016/j.cell.2007.12.018
Zhang R, Zhang LH, Xie X (2013) iPSCs and small molecules: a reciprocal effort towards better approaches for drug discovery. Acta Pharmacol Sin 34(6):765–776. doi:10.1038/aps.2013.21
Tsubokawa T, Yamaguchi-Okada M, Calvert JW, Solaroglu I, Shimamura N, Yata K, Zhang JH (2006) Neurovascular and neuronal protection by E64d after focal cerebral ischemia in rats. J Neurosci Res 84(4):832–840. doi:10.1002/jnr.20977
Latini L, Bisicchia E, Sasso V, Chiurchiu V, Cavallucci V, Molinari M, Maccarrone M, Viscomi MT (2014) Cannabinoid CB2 receptor (CB2R) stimulation delays rubrospinal mitochondrial-dependent degeneration and improves functional recovery after spinal cord hemisection by ERK1/2 inactivation. Cell Death Dis 5:e1404. doi:10.1038/cddis.2014.364
Chen HC, Fong TH, Lee AW, Chiu WT (2012) Autophagy is activated in injured neurons and inhibited by methylprednisolone after experimental spinal cord injury. Spine 37(6):470–475. doi:10.1097/BRS.0b013e318221e859
Hao HH, Wang L, Guo ZJ, Bai L, Zhang RP, Shuang WB, Jia YJ, Wang J, Li XY, Liu Q (2013) Valproic acid reduces autophagy and promotes functional recovery after spinal cord injury in rats. Neurosci Bull 29(4):484–492. doi:10.1007/s12264-013-1355-6
Sarkar C, Zhao Z, Aungst S, Sabirzhanov B, Faden AI, Lipinski MM (2014) Impaired autophagy flux is associated with neuronal cell death after traumatic brain injury. Autophagy 10(12):2208–2222. doi:10.4161/15548627.2014.981787
Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171(4):603–614. doi:10.1083/jcb.200507002
Lipinski MM, Zheng B, Lu T, Yan Z, Py BF, Ng A, Xavier RJ, Li C, Yankner BA, Scherzer CR, Yuan J (2010) Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer’s disease. Proc Natl Acad Sci U S A 107(32):14164–14169. doi:10.1073/pnas.1009485107
Knoferle J, Koch JC, Ostendorf T, Michel U, Planchamp V, Vutova P, Tonges L, Stadelmann C, Bruck W, Bahr M, Lingor P (2010) Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc Natl Acad Sci U S A 107(13):6064–6069. doi:10.1073/pnas.0909794107
Heckmann BL, Yang X, Zhang X, Liu J (2013) The autophagic inhibitor 3-methyladenine potently stimulates PKA-dependent lipolysis in adipocytes. Br J Pharmacol 168(1):163–171. doi:10.1111/j.1476-5381.2012.02110.x
Marinelli S, Nazio F, Tinari A, Ciarlo L, D’Amelio M, Pieroni L, Vacca V, Urbani A, Cecconi F, Malorni W, Pavone F (2014) The autophagic inhibitor 3-methyladenine potently stimulates PKA-dependent lipolysis in adipocytes. Pain 155(1):93–107. doi:10.1016/j.pain.2013.09.013
Acknowledgments
This work was supported by the International Research for Paraplegia (P141 to M.T.V.) and by the Italian Ministry of Health (Ricerca Corrente; to M. M.) and partially by the program Young Researchers of Italian Ministry of Health (GR-2010.2310524 to M.T.V.; GR-2011-02351457 to M.D.). The professional editorial work of Blue Pencil Science is also acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Elisa Bisicchia and Laura Latini contributed equally to this work.
Marcello D’Amelio and Maria Teresa Viscomi are equal senior authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Bisicchia, E., Latini, L., Cavallucci, V. et al. Autophagy Inhibition Favors Survival of Rubrospinal Neurons After Spinal Cord Hemisection. Mol Neurobiol 54, 4896–4907 (2017). https://doi.org/10.1007/s12035-016-0031-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0031-z