Abstract
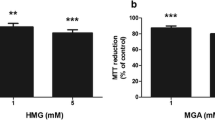
Patients affected by glutaric aciduria type I (GA-I) show progressive cortical leukoencephalopathy whose pathogenesis is poorly known. In the present work, we exposed cortical astrocytes of wild-type (Gcdh +/+) and glutaryl-CoA dehydrogenase knockout (Gcdh −/−) mice to the oxidative stress inducer menadione and measured mitochondrial bioenergetics, redox homeostasis, and cell viability. Mitochondrial function (MTT and JC1-mitochondrial membrane potential assays), redox homeostasis (DCFH oxidation, nitrate and nitrite production, GSH concentrations and activities of the antioxidant enzymes SOD and GPx), and cell death (propidium iodide incorporation) were evaluated in primary cortical astrocyte cultures of Gcdh +/+ and Gcdh −/− mice unstimulated and stimulated by menadione. We also measured the pro-inflammatory response (TNFα levels, IL1-β and NF-ƙB) in unstimulated astrocytes obtained from these mice. Gcdh −/− mice astrocytes were more vulnerable to menadione-induced oxidative stress (decreased GSH concentrations and altered activities of the antioxidant enzymes), mitochondrial dysfunction (decrease of MTT reduction and JC1 values), and cell death as compared with Gcdh +/+ astrocytes. A higher inflammatory response (TNFα, IL1-β and NF-ƙB) was also observed in Gcdh −/− mice astrocytes. These data indicate a higher susceptibility of Gcdh −/− cortical astrocytes to oxidative stress and mitochondrial dysfunction, probably leading to cell death. It is presumed that these pathomechanisms may contribute to the cortical leukodystrophy observed in GA-I patients.








Similar content being viewed by others
References
Goodman SI, Frerman F (2001) Organic acidemias due to defects in lysine oxidation: 2-ketoadipic academia and glutaric acidemia. The metabolic and molecular bases of inherited disease, 8 edn. McGraw-Hill, New York
Goodman SI, Norenberg MD, Shikes RH, Breslich DJ, Moe PG (1977) Glutaric aciduria: biochemical and morphologic considerations. J Pediatr 90(5):746–750
Funk CB, Prasad AN, Frosk P, Sauer S, Kolker S, Greenberg CR, Del Bigio MR (2005) Neuropathological, biochemical and molecular findings in a glutaric acidemia type 1 cohort. Brain 128(Pt 4):711–722
Hoffmann GF, Zschocke J (1999) Glutaric aciduria type I: from clinical, biochemical and molecular diversity to successful therapy. J Inherit Metab Dis 22(4):381–391
Busquets C, Coll MJ, Merinero B, Ugarte M, Ruiz MA, Martinez Bermejo A, Ribes A (2000) Prenatal molecular diagnosis of glutaric aciduria type I by direct mutation analysis. Prenat Diagn 20(9):761–764
Koeller DM, Woontner M, Crnic LS, Kleinschmidt-DeMasters B, Stephens J, Hunt EL, Goodman SI (2002) Biochemical, pathologic and behavioral analysis of a mouse model of glutaric acidemia type I. Hum Mol Genet 11(4):347–357
Koeller DM, Sauer S, Wajner M, de Mello CF, Goodman SI, Woontner M, Muhlhausen C, Okun JG, et al. (2004) Animal models for glutaryl-CoA dehydrogenase deficiency. J Inherit Metab Dis 27(6):813–818
Zinnanti WJ, Lazovic J, Wolpert EB, Antonetti DA, Smith MB, Connor JR, Woontner M, Goodman SI, et al. (2006) A diet-induced mouse model for glutaric aciduria type I. Brain 129(Pt 4):899–910
Zinnanti WJ, Lazovic J, Housman C, LaNoue K, O'Callaghan JP, Simpson I, Woontner M, Goodman SI, et al. (2007) Mechanism of age-dependent susceptibility and novel treatment strategy in glutaric acidemia type I. J Clin Invest 117(11):3258–3270
Kolker S, Ahlemeyer B, Krieglstein J, Hoffmann GF (1999) 3-hydroxyglutaric and glutaric acids are neurotoxic through NMDA receptors in vitro. J Inherit Metab Dis 22(3):259–262
Kolker S, Ahlemeyer B, Krieglstein J, Hoffmann GF (2000) Cerebral organic acid disorders induce neuronal damage via excitotoxic organic acids in vitro. Amino Acids 18(1):31–40
Lamp J, Keyser B, Koeller DM, Ullrich K, Braulke T, Muhlhausen C (2011) Glutaric aciduria type 1 metabolites impair the succinate transport from astrocytic to neuronal cells. J Biol Chem 286(20):17777–17784
Olivera S, Fernandez A, Latini A, Rosillo JC, Casanova G, Wajner M, Cassina P, Barbeito L (2008) Astrocytic proliferation and mitochondrial dysfunction induced by accumulated glutaric acidemia I (GAI) metabolites: possible implications for GAI pathogenesis. Neurobiol Dis 32(3):528–534
Olivera-Bravo S, Fernandez A, Sarlabos MN, Rosillo JC, Casanova G, Jimenez M, Barbeito L (2011) Neonatal astrocyte damage is sufficient to trigger progressive striatal degeneration in a rat model of glutaric acidemia-I. PLoS One 6(6):e20831
Olivera-Bravo S, Isasi E, Fernandez A, Rosillo JC, Jimenez M, Casanova G, Sarlabos MN, Barbeito L (2014) White matter injury induced by perinatal exposure to glutaric acid. Neurotox Res 25(4):381–391
Jafari P, Braissant O, Zavadakova P, Henry H, Bonafe L, Ballhausen D (2013) Ammonium accumulation and cell death in a rat 3D brain cell model of glutaric aciduria type I. PLoS One 8(1):e53735
Isasi E, Barbeito L, Olivera-Bravo S (2014) Increased blood-brain barrier permeability and alterations in perivascular astrocytes and pericytes induced by intracisternal glutaric acid. Fluids Barriers CNS 11:15
Belanger M, Magistretti PJ (2009) The role of astroglia in neuroprotection. Dialogues Clin Neurosci 11(3):281–295
Olivera-Bravo S, Barbeito L (2015) A role of astrocytes in mediating postnatal neurodegeneration in glutaric acidemia-type 1. FEBS Lett 589(22):3492–3497
Olivera-Bravo S, Ribeiro CA, Isasi E, Trias E, Leipnitz G, Diaz-Amarilla P, Woontner M, Beck C, et al. (2015) Striatal neuronal death mediated by astrocytes from the gcdh−/− mouse model of glutaric acidemia type I. Hum Mol Genet 24(16):4504–4515
Kolker S, Ahlemeyer B, Krieglstein J, Hoffmann GF (2000) Methylmalonic acid induces excitotoxic neuronal damage in vitro. J Inherit Metab Dis 23(4):355–358
Wajner M, Latini A, Wyse AT, Dutra-Filho CS (2004) The role of oxidative damage in the neuropathology of organic acidurias: insights from animal studies. J Inherit Metab Dis 27(4):427–448
Rosa RB, Dalcin KB, Schmidt AL, Gerhardt D, Ribeiro CA, Ferreira GC, Schuck PF, Wyse AT, et al. (2007) Evidence that glutaric acid reduces glutamate uptake by cerebral cortex of infant rats. Life Sci 81(25–26):1668–1676
Ferreira Gda C, Viegas CM, Schuck PF, Tonin A, Ribeiro CA, Coelho Dde M, Dalla-Costa T, Latini A, et al. (2005) Glutaric acid administration impairs energy metabolism in midbrain and skeletal muscle of young rats. Neurochem Res 30(9):1123–1131
Ferreira GC, Tonin A, Schuck PF, Viegas CM, Ceolato PC, Latini A, Perry ML, Wyse AT, et al. (2007) Evidence for a synergistic action of glutaric and 3-hydroxyglutaric acids disturbing rat brain energy metabolism. Int J Dev Neurosci 25(6):391–398
Ferreira Gda C, Schuck PF, Viegas CM, Tonin A, Latini A, Dutra-Filho CS, Wyse AT, Wannmacher CM, et al. (2007) Energy metabolism is compromised in skeletal muscle of rats chronically-treated with glutaric acid. Metab Brain Dis 22(1):111–123
Latini A, Ferreira GC, Scussiato K, Schuck PF, Solano AF, Dutra-Filho CS, Vargas CR, Wajner M (2007) Induction of oxidative stress by chronic and acute glutaric acid administration to rats. Cell Mol Neurobiol 27(4):423–438
Seminotti B, da Rosa MS, Fernandes CG, Amaral AU, Braga LM, Leipnitz G, de Souza DO, Woontner M, et al. (2012) Induction of oxidative stress in brain of glutaryl-CoA dehydrogenase deficient mice by acute lysine administration. Mol Genet Metab.
Seminotti B, Amaral AU, da Rosa MS, Fernandes CG, Leipnitz G, Olivera-Bravo S, Barbeito L, Ribeiro CA, et al. (2013) Disruption of brain redox homeostasis in glutaryl-CoA dehydrogenase deficient mice treated with high dietary lysine supplementation. Mol Genet Metab 108(1):30–39
Seminotti B, Ribeiro RT, Amaral AU, da Rosa MS, Pereira CC, Leipnitz G, Koeller DM, Goodman S, et al. (2014) Acute lysine overload provokes protein oxidative damage and reduction of antioxidant defenses in the brain of infant glutaryl-CoA dehydrogenase deficient mice: a role for oxidative stress in GA I neuropathology. J Neurol Sci 344(1–2):105–113
Amaral AU, Cecatto C, Seminotti B, Zanatta A, Fernandes CG, Busanello EN, Braga LM, Ribeiro CA, et al. (2012) Marked reduction of Na(+), K(+)-ATPase and creatine kinase activities induced by acute lysine administration in glutaryl-CoA dehydrogenase deficient mice. Mol Genet Metab 107(1–2):81–86
Amaral AU, Seminotti B, Cecatto C, Fernandes CG, Busanello EN, Zanatta A, Kist LW, Bogo MR, et al. (2012) Reduction of Na+, K + -ATPase activity and expression in cerebral cortex of glutaryl-CoA dehydrogenase deficient mice: a possible mechanism for brain injury in glutaric aciduria type I. Mol Genet Metab 107(3):375–382
Amaral AU, Cecatto C, Seminotti B, Ribeiro CA, Lagranha VL, Pereira CC, de Oliveira FH, de Souza DG, et al. (2015) Experimental evidence that bioenergetics disruption is not mainly involved in the brain injury of glutaryl-CoA dehydrogenase deficient mice submitted to lysine overload. Brain Res 1620:116–129
Loor G, Kondapalli J, Schriewer JM, Chandel NS, Vanden Hoek TL, Schumacker PT (2010) Menadione triggers cell death through ROS-dependent mechanisms involving PARP activation without requiring apoptosis. Free Radic Biol Med 49(12):1925–1936
Pedersen CB, Zolkipli Z, Vang S, Palmfeldt J, Kjeldsen M, Stenbroen V, Schmidt SP, Wanders RJ, et al. (2010) Antioxidant dysfunction: potential risk for neurotoxicity in ethylmalonic aciduria. J Inherit Metab Dis 33(3):211–222
Zanatta A, Rodrigues MD, Amaral AU, Souza DG, Quincozes-Santos A, Wajner M (2016) Ornithine and Homocitrulline impair mitochondrial function, decrease antioxidant defenses and induce cell death in menadione-stressed rat cortical astrocytes: potential mechanisms of neurological dysfunction in HHH syndrome. Neurochem Res. In press.
Zolkipli Z, Pedersen CB, Lamhonwah AM, Gregersen N, Tein I (2011) Vulnerability to oxidative stress in vitro in pathophysiology of mitochondrial short-chain acyl-CoA dehydrogenase deficiency: response to antioxidants. PLoS One 6(4):e17534
Gyetvai A, Emri T, Fekete A, Varga Z, Gazdag Z, Pesti M, Belagyi J, Emody L, et al. (2007) High-dose methylprednisolone influences the physiology and virulence of Candida albicans ambiguously and enhances the candidacidal activity of the polyene antibiotic amphotericin B and the superoxide-generating agent menadione. FEMS Yeast Res 7(2):265–275
Liu Y, Song Y, De Pascali F, Liu X, Villamena FA, Zweier JL (2012) Tetrathiatriarylmethyl radical with a single aromatic hydrogen as a highly sensitive and specific superoxide probe. Free Radic Biol Med 53(11):2081–2091
dos Santos AQ, Nardin P, Funchal C, de Almeida LM, Jacques-Silva MC, Wofchuk ST, Goncalves CA, Gottfried C (2006) Resveratrol increases glutamate uptake and glutamine synthetase activity in C6 glioma cells. Arch Biochem Biophys 453(2):161–167
Bobermin LD, Quincozes-Santos A, Guerra MC, Leite MC, Souza DO, Goncalves CA, Gottfried C (2012) Resveratrol prevents ammonia toxicity in astroglial cells. PLoS One 7(12):e52164
Reers M, Smiley ST, Mottola-Hartshorn C, Chen A, Lin M, Chen LB (1995) Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol 260:406–417
Quincozes-Santos A, Nardin P, de Souza DF, Gelain DP, Moreira JC, Latini A, Goncalves CA, Gottfried C (2009) The janus face of resveratrol in astroglial cells. Neurotox Res 16(1):30–41
Quincozes-Santos A, Bobermin LD, Latini A, Wajner M, Souza DO, Goncalves CA, Gottfried C (2013) Resveratrol protects C6 astrocyte cell line against hydrogen peroxide-induced oxidative stress through heme oxygenase 1. PLoS One 8(5):e64372
Souza DG, Bellaver B, Souza DO, Quincozes-Santos A (2013) Characterization of adult rat astrocyte cultures. PLoS One 8(3):e60282
Marklund SL (1985) Pyrogallol autoxidation. Handbook for oxygen radical research. CRC Press, Boca Raton, FL, pp. 243–247
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:7
Lowry OH, Rosebrough NJ, Farr AL, Randal RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Seminotti B, da Rosa MS, Fernandes CG, Amaral AU, Braga LM, Leipnitz G, de Souza DO, Woontner M, et al. (2012) Induction of oxidative stress in brain of glutaryl-CoA dehydrogenase deficient mice by acute lysine administration. Mol Genet Metab 106(1):31–38
Busanello EN, Fernandes CG, Martell RV, Lobato VG, Goodman S, Woontner M, de Souza DO, Wajner M (2014) Disturbance of the glutamatergic system by glutaric acid in striatum and cerebral cortex of glutaryl-CoA dehydrogenase-deficient knockout mice: possible implications for the neuropathology of glutaric acidemia type I. J Neurol Sci 346(1–2):260–267
Lagranha VL, Matte U, de Carvalho TG, Seminotti B, Pereira CC, Koeller DM, Woontner M, Goodman SI, et al. (2014) Increased glutamate receptor and transporter expression in the cerebral cortex and striatum of gcdh-/- mice: possible implications for the neuropathology of glutaric acidemia type I. PLoS One 9(3):e90477
Rodrigues MDN, Seminotti B, Amaral UA, Leipnitz G, Goodman SI, Woontner M, Souza DOG, Wajner M (2015) Experimental evidence that overexpression of NR2B glutamate receptor subunit is associated with brain vacuolation in adult glutaryl-CoA dehydrogenase deficient mice: a potential role for glutamatergic-induced excitotoxicity in GA I neuropathology. J Neurol Sci 359(1–2):133–140
Superti-Furga A, Hoffmann GF (1997) Glutaric aciduria type 1 (glutaryl-CoA-dehydrogenase deficiency): advances and unanswered questions. Report from an international meeting. Eur J Pediatr 156(11):821–828
Kolker S, Ahlemeyer B, Krieglstein J, Hoffmann GF (2000) Evaluation of trigger factors of acute encephalopathy in glutaric aciduria type I: fever and tumour necrosis factor-alpha. J Inherit Metab Dis 23(4):359–362
Ullrich K, Flott-Rahmel B, Schluff P, Musshoff U, Das A, Lucke T, Steinfeld R, Christensen E, et al. (1999) Glutaric aciduria type I: pathomechanisms of neurodegeneration. J Inherit Metab Dis 22(4):392–403
Strauss KA, Morton DH (2003) Type I glutaric aciduria, part 2: a model of acute striatal necrosis. Am J Med Genet C Semin Med Genet 121C(1):53–70. doi:10.1002/ajmg.c.20008
Sauer SW, Okun JG, Schwab MA, Crnic LR, Hoffmann GF, Goodman SI, Koeller DM, Kolker S (2005) Bioenergetics in glutaryl-coenzyme A dehydrogenase deficiency: a role for glutaryl-coenzyme A. J Biol Chem 280(23):21830–21836
Kolker S, Koeller DM, Sauer S, Horster F, Schwab MA, Hoffmann GF, Ullrich K, Okun JG (2004) Excitotoxicity and bioenergetics in glutaryl-CoA dehydrogenase deficiency. J Inherit Metab Dis 27(6):805–812
Coyle JT, Puttfarcken P (1993) Oxidative stress, glutamate, and neurodegenerative disorders. Science 262(5134):689–695
Beal MF (2000) Energetics in the pathogenesis of neurodegenerative diseases. Trends Neurosci 23(7):298–304
Frantseva MV, Perez Velazquez JL, Tsoraklidis G, Mendonca AJ, Adamchik Y, Mills LR, Carlen PL, Burnham MW (2000) Oxidative stress is involved in seizure-induced neurodegeneration in the kindling model of epilepsy. Neuroscience 97(3):431–435
Halliwell B, Gutteridge JMC (2007) Cellular responses to oxidative stress: adaptation, damage, repair, senescence and death. In: Halliwell B, Gutteridge JMC (eds) Free radicals in biology and medicine. Oxford University Press Inc., Oxford, pp. 187–340
Halliwell B (1992) Reactive oxygen species and the central nervous system. J Neurochem 59(5):1609–1623
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97(6):1634–1658
Dringen R, Kussmaul L, Gutterer JM, Hirrlinger J, Hamprecht B (1999) The glutathione system of peroxide detoxification is less efficient in neurons than in astroglial cells. J Neurochem 72(6):2523–2530
Siu AW, Reiter RJ, To CH (1998) The efficacy of vitamin E and melatonin as antioxidants against lipid peroxidation in rat retinal homogenates. J Pineal Res 24(4):239–244
Gloire G, Legrand-Poels S, Piette J (2006) NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72(11):1493–1505
Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW (2010) When NRF2 talks, who's listening? Antioxid Redox Signal 13(11):1649–1663
Satoh T, Lipton SA (2007) Redox regulation of neuronal survival mediated by electrophilic compounds. Trends Neurosci 30(1):37–45
Bermejo P, Martin-Aragon S, Benedi J, Susin C, Felici E, Gil P, Ribera JM, Villar AM (2008) Peripheral levels of glutathione and protein oxidation as markers in the development of Alzheimer's disease from mild cognitive impairment. Free Radic Res 42(2):162–170
Rosales-Corral S, Reiter RJ, Tan DX, Ortiz GG, Lopez-Armas G (2010) Functional aspects of redox control during neuroinflammation. Antioxid Redox Signal 13(2):193–247
Double KL, Reyes S, Werry EL, Halliday GM (2010) Selective cell death in neurodegeneration: why are some neurons spared in vulnerable regions? Prog Neurobiol 92(3):316–329
Farooqui AA (2010) Studies on plasmalogen-selective phospholipase A2 in brain. Mol Neurobiol 41(2–3):267–273
Farooqui T, Farooqui AA (2011) Lipid-mediated oxidative stress and inflammation in the pathogenesis of Parkinson's disease. Parkinsons Dis 2011:247467
Kolker S, Sauer SW, Okun JG, Hoffmann GF, Koeller DM (2006) Lysine intake and neurotoxicity in glutaric aciduria type I: towards a rationale for therapy? Brain 129(Pt 8):e54
Sauer SW, Okun JG, Fricker G, Mahringer A, Muller I, Crnic LR, Muhlhausen C, Hoffmann GF, et al. (2006) Intracerebral accumulation of glutaric and 3-hydroxyglutaric acids secondary to limited flux across the blood-brain barrier constitute a biochemical risk factor for neurodegeneration in glutaryl-CoA dehydrogenase deficiency. J Neurochem 97(3):899–910
Acknowledgments
We are grateful to the financial support of CNPq - 470236/2012-4, FAPERGS—10/0031-1, PROPESQ/UFRGS—PIBIC 27613, FINEP/IBN-Net—01.06.0842-00 and INCT-EN—573677/2008-5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Statement
This study was performed in strict accordance with the Principles of Laboratory Animal Care, National Institute of Health of United States of America, NIH, publication n° 85–23, revised in 2011, and approved by the Ethical Committee for the Care and Use of Laboratory Animals of Hospital de Clínicas de Porto Alegre.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rodrigues, M.D.N., Seminotti, B., Zanatta, Â. et al. Higher Vulnerability of Menadione-Exposed Cortical Astrocytes of Glutaryl-CoA Dehydrogenase Deficient Mice to Oxidative Stress, Mitochondrial Dysfunction, and Cell Death: Implications for the Neurodegeneration in Glutaric Aciduria Type I. Mol Neurobiol 54, 4795–4805 (2017). https://doi.org/10.1007/s12035-016-0023-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0023-z