Abstract
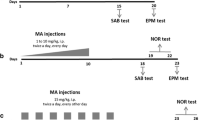
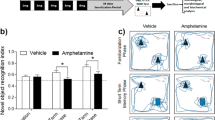
(±)3,4-Methylenedioxymethamphetamine (MDMA) is a relatively selective dopaminergic neurotoxin in mice. This study was designed to evaluate whether MDMA exposure affects their recognition memory and hippocampal expression of plasticity markers. Mice were administered with increasing doses of MDMA once per week for 8 weeks (three times in 1 day, every 3 h) and killed 2 weeks (2w) or 3 months (3m) later. The treatment did not modify hippocampal tryptophan hydroxylase 2, a serotonergic indicator, but induced an initial reduction in dopaminergic markers in substantia nigra, which remained stable for at least 3 months. In parallel, MDMA produced a decrease in dopamine (DA) levels in the striatum at 2w, which were restored 3 months later, suggesting dopaminergic terminal regeneration (sprouting phenomenon). Moreover, recognition memory was assessed using the object recognition test. Young (2w) and mature (3m) adult mice exhibited impaired memory after 24-h but not after just 1-h retention interval. Two weeks after the treatment, animals showed constant levels of CREB but an increase in its phosphorylated form and in c-Fos expression. Brain-derived neurotrophic factor (BDNF) and especially Arc overexpression was sustained and long-lasting. We cannot rule out the absence of MDMA injury in the hippocampus being due to the generation of BDNF. The levels of NMDAR2B, PSD-95, and synaptophysin were unaffected. In conclusion, the young mice exposed to MDMA showed increased expression of early key markers of plasticity, which sometimes remained for 3 months, and suggests hippocampal maladaptive plasticity that could explain memory deficits evidenced here.








Similar content being viewed by others
References
Pubill D, Canudas AM, Pallàs M, Camins A, Camarasa J, Escubedo E (2003) Different glial response to methamphetamine- and methylenedioxymethamphetamine-induced neurotoxicity. Naunyn Schmiedeberg’s Arch Pharmacol 367:490–499
Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI (2003) The Pharmacology and Clinical Pharmacology of 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”). Pharmacol Rev 55:463–508
Logan BJ, Laverty R, Sanderson WD, Yee YB (1988) Differences between rats and mice in MDMA (methylenedioxymethylamphetamine) neurotoxicity. Eur J Pharmacol 152:227–234
Chipana C, Camarasa J, Pubill D, Escubedo E (2006) Protection against MDMA-induced dopaminergic neurotoxicity in mice by methyllycaconitine: involvement of nicotinic receptors. Neuropharmacology 51:885–895. doi:10.1016/j.neuropharm.2006.05.032
O’Callaghan JP, Miller DB (1994) Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther 270:741–751
Colado MI, Camarero J, Mechan AO, Sanchez V, Esteban B, Elliott JM, Green AR (2001) A study of the mechanisms involved in the neurotoxic action of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) on dopamine neurones in mouse brain. Br J Pharmacol 134:1711–1723
O’Shea E, Esteban B, Camarero J, Green AR, Colado MI (2001) Effect of GBR 12909 and fluoxetine on the acute and long term changes induced by MDMA (“ecstasy”) on the 5-HT and dopamine concentrations in mouse brain. Neuropharmacology 40:65–74
Able JA, Gudelsky GA, Vorhees CV, Williams MT (2006) 3,4-Methylenedioxymethamphetamine in adult rats produces deficits in path integration and spatial reference memory. Biol Psychiatry 59:1219–1226
Sprague JE, Preston AS, Leifheit M, Woodside B (2003) Hippocampal serotonergic damage induced by MDMA (ecstasy): effects on spatial learning. Physiol Behav 79:281–287
Vorhees CV, Reed TM, Skelton MR, Williams MT (2004) Exposure to 3,4-methylenedioxymethamphetamine (MDMA) on postnatal days 11–20 induces reference but not working memory deficits in the Morris water maze in rats: implications of prior learning. Int J Dev Neurosci Off J Int Soc Dev Neurosci 22:247–259
Morley KC, Gallate JE, Hunt GE, Mallet PE, McGregor IS (2001) Increased anxiety and impaired memory in rats 3 months after administration of 3,4-methylenedioxymethamphetamine (“ecstasy”). Eur J Pharmacol 433:91–99
Salzmann J, Marie-claire C, Guen SL, Roques BP, Noble F (2003) Importance of ERK activation in behavioral and biochemical effects induced by MDMA in mice. Br J Pharmacol 140:831–838
Tamburini I, Blandini F, Gesi M, Frenzilli G, Nigro M, Giusiani M, Paparelli A, Fornai F (2006) MDMA induces caspase-3 activation in the limbic system but not in striatum. Ann N Y Acad Sci 1074:377–381
Busceti CL, Biagioni F, Riozzi B, Battaglia G, Storto M, Cinque C, Molinaro G, Gradini R et al (2008) Enhanced tau phosphorylation in the hippocampus of mice treated with 3,4-methylenedioxymethamphetamine (“Ecstasy”). J Neurosci 28:3234–3245
Robinson TE, Kolb B (2004) Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47(Supplement 1):33–46
Nestler EJ (2001) Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2:119–128
Ball KT, Wellman CL, Fortenberry E, Rebec GV (2009) Sensitizing regimens of (+/−)3, 4-methylenedioxymethamphetamine (ecstasy) elicit enduring and differential structural alterations in the brain motive circuit of the rat. Neuroscience 160:264–274
Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes C (2000) Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci Off J Soc Neurosci 20:3993–4001
Kodama M, Akiyama K, Ujike H, Shimizu Y, Tanaka Y, Kuroda S (1998) A robust increase in expression of arc gene, an effector immediate early gene, in the rat brain after acute and chronic methamphetamine administration. Brain Res 796:273–283
Pei Q, Lewis L, Sprakes ME, Jones EJ, Grahame-Smith DG, Zetterström TS (2000) Serotonergic regulation of mRNA expression of Arc, an immediate early gene selectively localized at neuronal dendrites. Neuropharmacology 39:463–470
Steward O, Worley PF (2001) Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron 30:227–240
Kalivas PW, O’Brien C (2007) Drug Addiction as a Pathology of Staged Neuroplasticity. Neuropsychopharmacology 33:166–180
Bramham CR, Messaoudi E (2005) BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol 76:99–125. doi:10.1016/j.pneurobio.2005.06.003
Broening HW, Morford LL, Inman-Wood SL, Fukumura M, Vorhees CV (2001) 3,4-methylenedioxymethamphetamine (ecstasy)-induced learning and memory impairments depend on the age of exposure during early development. J Neurosci Off J Soc Neurosci 21:3228–3235
Hammersley R, Ditton J, Smith I, Short E (1999) Patterns of ecstasy use by drug users. Br J Criminol 39:625–647
Spanos LJ, Yamamoto BK (1989) Acute and subchronic effects of methylenedioxymethamphetamine [(+/−)MDMA] on locomotion and serotonin syndrome behavior in the rat. Pharmacol Biochem Behav 32:835–840
Abad S, Fole A, del Olmo N, Pubill D, Pallàs M, Junyent F, Camarasa J, Camins A et al (2014) MDMA enhances hippocampal-dependent learning and memory under restrictive conditions, and modifies hippocampal spine density. Psychopharmacology (Berlin) 231:863–874
Paxinos G, Franklin KBJ (2001) Mouse brain in stereotaxic coordinates
Pedrós I, Petrov D, Allgaier M, Sureda F, Barroso E, Beas-Zarate C, Auladell C, Pallàs M et al (2014) Early alterations in energy metabolism in the hippocampus of APPswe/PS1dE9 mouse model of Alzheimer’s disease. Biochim Biophys Acta 1842:1556–1566
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods San Diego Calif 25:402–408. doi:10.1006/meth.2001.1262
Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ (1995) Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci Off J Soc Neurosci 15:61–69
Rothman RB, Baumann MH (2003) Monoamine transporters and psychostimulant drugs. Eur J Pharmacol 479:23–40
Nash JF, Roth BL, Brodkin JD, Nichols DE, Gudelsky GA (1994) Effect of the R(−) and S(+) isomers of MDA and MDMA on phosphatidyl inositol turnover in cultured cells expressing 5-HT2A or 5-HT2C receptors. Neurosci Lett 177:111–115
Battaglia G, Yeh SY, De Souza EB (1988) MDMA-induced neurotoxicity: parameters of degeneration and recovery of brain serotonin neurons. Pharmacol Biochem Behav 29:269–274
Scanzello CR, Hatzidimitriou G, Martello AL, Katz JL, Ricaurte GA (1993) Serotonergic recovery after (+/−)3,4-(methylenedioxy) methamphetamine injury: observations in rats. J Pharmacol Exp Ther 264:1484–1491
Fischer C, Hatzidimitriou G, Wlos J, Katz J, Ricaurte G (1995) Reorganization of ascending 5-HT axon projections in animals previously exposed to the recreational drug (+/−)3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). J Neurosci Off J Soc Neurosci 15:5476–5485
Costa G, Frau L, Wardas J, Pinna A, Plumitallo A, Morelli M (2013) MPTP-induced dopamine neuron degeneration and glia activation is potentiated in MDMA-pretreated mice. Mov Disord 28:1957–1965
Schmidt CJ, Abbate GM, Black CK, Taylor VL (1990) Selective 5-hydroxytryptamine2 receptor antagonists protect against the neurotoxicity of methylenedioxymethamphetamine in rats. J Pharmacol Exp Ther 255:478–483
Granado N, O’Shea E, Bove J, Vila M, Colado MI, Moratalla R (2008) Persistent MDMA-induced dopaminergic neurotoxicity in the striatum and substantia nigra of mice. J Neurochem 107:1102–1112
Hammond RS, Tull LE, Stackman RW (2004) On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem 82:26–34
Schenk S (2011) MDMA (“ecstasy”) abuse as an example of dopamine neuroplasticity. Neurosci Biobehav Rev 35:1203–1218
Williams MT, Skelton MR, Longacre ID, Huggins KN, Maple AM, Vorhees CV, Brown RW (2014) Neuronal reorganization in adult rats neonatally exposed to (±)-3,4-methylenedioxymethamphetamine. Toxicol Rep 1:699–706
van Nieuwenhuijzen PS, Kashem MA, Matsumoto I, Hunt GE, McGregor IS (2010) A long hangover from party drugs: residual proteomic changes in the hippocampus of rats 8 weeks after γ-hydroxybutyrate (GHB), 3,4-methylenedioxymethamphetamine (MDMA) or their combination. Neurochem Int 56:871–877
Silva AJ, Kogan JH, Frankland PW, Kida S (1998) CREB and memory. Annu Rev Neurosci 21:127–148. doi:10.1146/annurev.neuro.21.1.127
Ying S-W, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR (2002) Brain-Derived Neurotrophic Factor Induces Long-Term Potentiation in Intact Adult Hippocampus: Requirement for ERK Activation Coupled to CREB and Upregulation of Arc Synthesis. J Neurosci 22:1532–1540
Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, Panja D, Schubert M et al (2009) The Arc of synaptic memory. Exp Brain Res 200:125–140
Huang F, Chotiner JK, Steward O (2007) Actin polymerization and ERK phosphorylation are required for Arc/Arg3.1 mRNA targeting to activated synaptic sites on dendrites. J Neurosci Off J Soc Neurosci 27:9054–9067. doi:10.1523/JNEUROSCI.2410-07.2007
Messaoudi E, Kanhema T, Soulé J, Tiron A, Dagyte G, da Silva B, Bramham CR (2007) Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci 27:10445–10455
Farris S, Lewandowski G, Cox CD, Steward O (2014) Selective localization of arc mRNA in dendrites involves activity- and translation-dependent mRNA degradation. J Neurosci Off J Soc Neurosci 34:4481–4493
Fosnaugh JS, Bhat RV, Yamagata K, Yamagata K, Worley PF, Baraban JM (1995) Activation of arc, a putative “effector” immediate early gene, by cocaine in rat brain. J Neurochem 64:2377–2380
Freeman WM, Brebner K, Lynch WJ, Patel KM, Robertson DJ, Roberts DC, Vrana KE (2002) Changes in rat frontal cortex gene expression following chronic cocaine. Brain Res Mol Brain Res 104:11–20
Caffino L, Giannotti G, Malpighi C, Racagni G, Filip M, Fumagalli F (2014) Long-term abstinence from developmental cocaine exposure alters Arc/Arg3.1 modulation in the rat medial prefrontal cortex. Neurotox Res 26:299–306
Huang CC, Yeh CM, Wu MY, Chang AY, Chan JY, Chan SH, Hsu KS (2011) Cocaine withdrawal impairs metabotropic glutamate receptor-dependent long-term depression in the nucleus accumbens. J Neurosci 31:4194–4203
Martínez-Turrillas R, Moyano S, Del Río J, Frechilla D (2006) Differential effects of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) on BDNF mRNA expression in rat frontal cortex and hippocampus. Neurosci Lett 402:126–130
Lindefors N, Ballarin M, Ernfors P, Falkenberg T, Persson H (1992) Stimulation of glutamate receptors increases expression of brain-derived neurotrophic factor mRNA in rat hippocampus. Ann N Y Acad Sci 648:296–299
Lauterborn JC, Lynch G, Vanderklish P, Arai A, Gall CM (2000) Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J Neurosci Off J Soc Neurosci 20:8–21
Zafra F, Lindholm D, Castren E, Hartikka J, Thoenen H (1992) Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J Neurosci Off J Soc Neurosci 12:4793–4799
Sano K, Nanba H, Tabuchi A, Tsuchiya T, Tsuda M (1996) BDNF gene can Be activated by Ca2+ signals without involvement of de novo AP-1 synthesis. Biochem Biophys Res Commun 229:788–793. doi:10.1006/bbrc.1996.1881
Abad S, Junyent F, Auladell C, Pubill D, Pallàs M, Camarasa J, Escubedo E, Camins A (2014) 3,4-Methylenedioxymethamphetamine enhances kainic acid convulsive susceptibility. Prog Neuropsychopharmacol Biol Psychiatry 54:231–242. doi:10.1016/j.pnpbp.2014.06.007
Steward O, Schuman EM (2001) Protein synthesis at synaptic sites on dendrites. Annu Rev Neurosci 24:299–325. doi:10.1146/annurev.neuro.24.1.299
Fumagalli F, Moro F, Caffino L, Orrù A, Cassina C, Giannotti G, Di Clemente A, Racagni G et al (2013) Region-specific effects on BDNF expression after contingent or non-contingent cocaine i.v. self-administration in rats. Int J Neuropsychopharmacol 16:913–918. doi:10.1017/S146114571200096X
Adori C, Andó RD, Ferrington L, Szekeres M, Vas S, Kelly PA, Hunyady L, Bagdy G (2010) Elevated BDNF protein level in cortex but not in hippocampus of MDMA-treated Dark Agouti rats: a potential link to the long-term recovery of serotonergic axons. Neurosci Lett 478:56–60. doi:10.1016/j.neulet.2010.04.061
Ozawa S, Kamiya H, Tsuzuki K (1998) Glutamate receptors in the mammalian central nervous system. Prog Neurobiol 54:581–618
Kalivas PW (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10:561–572. doi:10.1038/nrn2515
Kindlundh-Högberg AMS, Blomqvist A, Malki R, Schiöth HB (2008) Extensive neuroadaptive changes in cortical gene-transcript expressions of the glutamate system in response to repeated intermittent MDMA administration in adolescent rats. BMC Neurosci 9:39
Acknowledgments
The authors thank J.A. Paulsen-Medalen for technical assistance, and they also acknowledge the Language Advisory Service of the University of Barcelona for editing the language of the manuscript. Sonia Abad received a fellowship from the Institut de Biomedicina (IBUB, University of Barcelona). Funding for this study was provided by the Plan Nacional sobre Drogas (2012I102) and the Ministerio de Economía y Competitividad (SAF 2013-46135-P) and by the Generalitat de Catalunya (2014SGR525). AC belongs to 2014SGR525, and JC, DP, and EE to 2014SGR1081.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abad, S., Camarasa, J., Pubill, D. et al. Adaptive Plasticity in the Hippocampus of Young Mice Intermittently Exposed to MDMA Could Be the Origin of Memory Deficits. Mol Neurobiol 53, 7271–7283 (2016). https://doi.org/10.1007/s12035-015-9618-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9618-z