Abstract
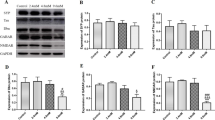
Lead exposure can cause cognitive dysfunction in children, thus it still raises important public health concerns in China and other countries. However, the underlying molecular mechanisms are still not well defined. In this study, we aimed to elucidate the mechanisms underlying lead neurotoxicity by focusing on alterations of synaptic proteins in the mouse hippocampus at the early life. Mother mice and their offspring were exposed to 0, 0.5, 1.0, and 2.0 g/L lead via drinking water from the first day of gestation until postnatal day (PND) 40. Synaptic ultrastructure and expressions of postsynaptic density protein-95 (PSD-95), neuronal nitric oxide synthase (nNOS) and synaptophysin (SYP) at both protein and gene levels in the hippocampus were analyzed. The results revealed that developmental lead exposure caused a diminished postsynaptic density in the hippocampus. Moreover, the protein levels of PSD-95, nNOS, and SYP decreased significantly due to developmental lead exposure. On the other hand, the messenger RNA (mRNA) levels of PSD-95 and SYP decreased significantly in PND 40 mice exposed to lead. Collectively, developmental lead exposure might result in decreased protein and gene expressions of both presynaptic and postsynaptic proteins. Our findings raised a possibility that alterations of synaptic proteins in the hippocampus induced by lead exposure at the early life might serve an important role for the subsequent intellectual impairments, e.g., deficits in spatial learning and memory ability at later ages shown in our recently published paper.










Similar content being viewed by others
References
Zhang S, Dai Y, Xie X, Fan Z, Tan Z, Zhang Y (2009) Surveillance of childhood blood lead levels in 14 cities of China in 2004–2006. Biomed Environ Sci 22(4):288–296
Liu H, Zhang J, Zheng P, Zhang Y (2005) Altered expression of MAP-2, GAP-43, and synaptophysin in the hippocampus of rats with chronic cerebral hypoperfusion correlates with cognitive impairment. Brain Res Mol Brain Res 139(1):169–177
Silva AJ (2003) Molecular and cellular cognitive studies of the role of synaptic plasticity in memory. J Neurobiol 54(1):224–237
Guilarte TR, McGlothan JL (2003) Selective decrease in NR1 subunit splice variant mRNA in the hippocampus of Pb2+-exposed rats: implications for synaptic targeting and cell surface expression of NMDAR complexes. Brain Res Mol Brain Res 113(1–2):37–43
Toscano CD, Guilarte TR (2005) Lead neurotoxicity: from exposure to molecular effects. Brain Res Brain Res Rev 49(3):529–554
Yu H, Li T, Cui Y, Liao Y, Wang G, Gao L, Zhao F, Jin Y (2014) Effects of lead exposure on D-serine metabolism in the hippocampus of mice at the early developmental stages. Toxicology 325:189–199
Carroll RC, Zukin RS (2002) NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci 25(11):571–577
Kornau H-C, Schenker LT, Kennedy MB, Seeburg PH (1995) Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 269(5231):1737–1740
Niethammer M, Kim E, Sheng M (1996) Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci 16(7):2157–2163
Christopherson KS, Hillier BJ, Lim WA, Bredt DS (1999) PSD-95 assembles a ternary complex with the N-Methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem 274(39):27467–27473
Jaffrey SR, Snowman AM, Eliasson MJ, Cohen NA, Snyder SH (1998) CAPON: a protein associated with neuronal nitric oxide synthase that regulates its interactions with PSD95. Neuron 20(1):115–124
Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M (1999) Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science 284(5421):1845–1848
Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O’Dell TJ, Grant SG (1998) Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 396(6710):433–439
Prast H, Philippu A (2001) Nitric oxide as modulator of neuronal function. Prog Neurobiol 64(1):51–68
Boehning D, Snyder SH (2003) Novel neural modulators. Annu Rev Neurosci 26:105–131
Bredt DS, Snyder SH (1990) Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A 87(2):682–685
Abu-Soud HM, Yoho LL, Stuehr DJ (1994) Calmodulin controls neuronal nitric-oxide synthase by a dual mechanism. Activation of intra- and interdomain electron transfer. J Biol Chem 269(51):32047–32050
Yan X, Song B, Zhang G (2004) Postsynaptic density protein 95 mediates Ca2+/calmodulin-dependent protein kinase II-activated serine phosphorylation of neuronal nitric oxide synthase during brain ischemia in rat hippocampus. Neurosci Lett 355(3):197–200
Garthwaite J (2008) Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci 27(11):2783–2802
Taqatqeh F, Mergia E, Neitz A, Eysel UT, Koesling D, Mittmann T (2009) More than a retrograde messenger: nitric oxide needs two cGMP pathways to induce hippocampal long-term potentiation. J Neurosci 29(29):9344–9350
Hopper RA, Garthwaite J (2006) Tonic and phasic nitric oxide signals in hippocampal long-term potentiation. J Neurosci 26(45):11513–11521
Arancio O, Kiebler M, Lee CJ, Lev-Ram V, Tsien RY, Kandel ER, Hawkins RD (1996) Nitric oxide acts directly in the presynaptic neuron to produce long-term potentiation in cultured hippocampal neurons. Cell 87(6):1025–1035
Greengard P, Valtorta F, Czernik AJ, Benfenati F (1993) Synaptic vesicle phosphoproteins and regulation of synaptic function. Science 259(5096):780–785
Valtorta F, Pennuto M, Bonanomi D, Benfenati F (2004) Synaptophysin: leading actor or walk-on role in synaptic vesicle exocytosis? Bioessays 26(4):445–453
Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR (2000) Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci 20(17):6587–6593
Schmitt U, Tanimoto N, Seeliger M, Schaeffel F, Leube RE (2009) Detection of behavioral alterations and learning deficits in mice lacking synaptophysin. Neuroscience 162(2):234–243
Rao JS, Kellom M, Kim HW, Rapoport SI, Reese EA (2012) Neuroinflammation and synaptic loss. Neurochem Res 37(5):903–910
Marrone DF, Petit TL (2002) The role of synaptic morphology in neural plasticity: structural interactions underlying synaptic power. Brain Res Brain Res Rev 38(3):291–308
Lynch MA (2004) Long-term potentiation and memory. Physiol Rev 84(1):87–136
Rabenstein RL, Addy NA, Caldarone BJ, Asaka Y, Gruenbaum LM, Peters LL, Gilligan DM, Fitzsimonds RM, Picciotto MR (2005) Impaired synaptic plasticity and learning in mice lacking beta-adducin, an actin-regulating protein. J Neurosci 25(8):2138–2145
Di G, Zheng Y (2013) Effects of high-speed railway noise on the synaptic ultrastructure and phosphorylated-CaMKII expression in the central nervous system of SD rats. Environ Toxicol Pharmacol 35(1):93–99
El-Husseini AE-D, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS (2000) PSD-95 involvement in maturation of excitatory synapses. Science 290(5495):1364–1368
Valtschanoff JG, Weinberg RJ (2001) Laminar organization of the NMDA receptor complex within the postsynaptic density. J Neurosci 21(4):1211–1217
Kellom M, Basselin M, Keleshian VL, Chen M, Rapoport SI, Rao JS (2012) Dose-dependent changes in neuroinflammatory and arachidonic acid cascade markers with synaptic marker loss in rat lipopolysaccharide infusion model of neuroinflammation. BMC Neurosci 13:50
Che Y, Tamatani M, Tohyama M (2000) Changes in mRNA for post-synaptic density-95 (PSD-95) and carboxy-terminal PDZ ligand of neuronal nitric oxide synthase following facial nerve transection. Brain Res Mol Brain Res 76(2):325–335
Rao A, Kim E, Sheng M, Craig AM (1998) Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture. J Neurosci 18(4):1217–1229
Neal AP, Worley PF, Guilarte TR (2011) Lead exposure during synaptogenesis alters NMDA receptor targeting via NMDA receptor inhibition. Neurotoxicology 32(2):281–289
Neal AP, Stansfield KH, Worley PF, Thompson RE, Guilarte TR (2010) Lead exposure during synaptogenesis alters vesicular proteins and impairs vesicular release: potential role of NMDA receptor-dependent BDNF signaling. Toxicol Sci 116(1):249–263
Chetty CS, Reddy GR, Murthy KS, Johnson J, Sajwan K, Desaiah D (2001) Perinatal lead exposure alters the expression of neuronal nitric oxide synthase in rat brain. Int J Toxicol 20(3):113–120
Nava-Ruíz C, Alcaraz-Zubeldia M, Méndez-Armenta M, Vergara P, Díaz-Ruìz A, Ríos C (2010) Nitric oxide synthase immunolocalization and expression in the rat hippocampus after sub-acute lead acetate exposure in rats. Exp Toxicol Pathol 62(3):311–316
Nava-Ruíz C, Méndez-Armenta M, Ríos C (2012) Lead neurotoxicity: effects on brain nitric oxide synthase. J Mol Histol 43(5):553–563
Wang H, Lu FM, Jin I, Udo H, Kandel ER, de Vente J, Walter U, Lohmann SM, Hawkins RD, Antonova I (2005) Presynaptic and postsynaptic roles of NO, cGK, and RhoA in long-lasting potentiation and aggregation of synaptic proteins. Neuron 45(3):389–403
Steinert JR, Kopp-Scheinpflug C, Baker C, Challiss RAJ, Mistry R, Haustein MD, Griffin SJ, Tong H, Graham BP, Forsythe ID (2008) Nitric oxide is a volume transmitter regulating postsynaptic excitability at a glutamatergic synapse. Neuron 60(4):642–656
Ota KT, Monsey MS, Wu MS, Schafe GE (2010) Synaptic plasticity and NO-cGMP-PKG signaling regulate pre- and postsynaptic alterations at rat lateral amygdale synapses following fear conditioning. PLoS One 5(6):e11236
Neal AP, Stansfield KH, Guilarte TR (2012) Enhanced nitric oxide production during lead (Pb2+) exposure recovers protein expression but not presynaptic localization of synaptic proteins in developing hippocampal neurons. Brain Res 1439:88–95
Acknowledgments
This work was supported by The National Natural Science Foundation of China (no. 31070992), Program for Liaoning Innovative Research Team in University (LT2015028), Liaoning Provincial Natural Science Foundation (no. 20102263), and Science and Technology Plan Project of Educational Department of Liaoning Province (no. L2010559).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declared no competing interests.
Rights and permissions
About this article
Cite this article
Yu, H., Liao, Y., Li, T. et al. Alterations of Synaptic Proteins in the Hippocampus of Mouse Offspring Induced by Developmental Lead Exposure. Mol Neurobiol 53, 6786–6798 (2016). https://doi.org/10.1007/s12035-015-9597-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9597-0