Abstract
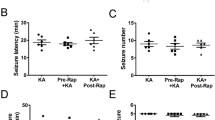
Germline and somatic mutations in key genes of the mammalian target of rapamycin (mTOR) pathway have been identified in seizure-associated disorders. mTOR mutations lead to aberrant activation of mTOR signaling, and, although affected neurons are critical for epileptogenesis, the role of mTOR activation in glial cells remains poorly understood. We previously reported a consistent activation of the mTOR pathway in astrocytes in the epileptic foci of temporal lobe epilepsy. In this study, it was demonstrated that mTOR deletion from reactive astrocytes prevents increases in seizure frequency over the disease course. By using a tamoxifen-inducible mTOR conditional knockout system and kainic acid, a model was developed that allowed astrocyte-specific mTOR gene deletion in mice with chronic epilepsy. Animals in which mTOR was deleted from 44 % of the astrocyte population exhibited a lower seizure frequency compared with controls. Down-regulation of mTOR significantly ameliorated astrogliosis in the sclerotic hippocampus but did not rescue mossy fiber sprouting. In cultured astrocytes, the mTOR pathway modulated the stability of the astroglial glutamate transporter 1 (Glt1) and influenced the ability of astrocytes to remove extracellular glutamate. Taken together, these data indicate that astrocytes with activated mTOR signaling may provide conditions that are favorable for spontaneous recurrent seizures.





Similar content being viewed by others
References
Costa-Mattioli M, Monteggia LM (2013) mTOR complexes in neurodevelopmental and neuropsychiatric disorders. Nat Neurosci 16(11):1537–1543. doi:10.1038/nn.3546
Pun RY, Rolle IJ, Lasarge CL, Hosford BE, Rosen JM, Uhl JD, Schmeltzer SN, Faulkner C et al (2012) Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron 75(6):1022–1034. doi:10.1016/j.neuron.2012.08.002
Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ, Yamada K, Gutmann DH (2002) Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol 52(3):285–296. doi:10.1002/ana.10283
Way SW, McKenna J 3rd, Mietzsch U, Reith RM, Wu HC, Gambello MJ (2009) Loss of Tsc2 in radial glia models the brain pathology of tuberous sclerosis complex in the mouse. Hum Mol Genet 18(7):1252–1265. doi:10.1093/hmg/ddp025
Kwasnicki A, Jeevan D, Braun A, Murali R, Jhanwar-Uniyal M (2015) Involvement of mTOR signaling pathways in regulating growth and dissemination of metastatic brain tumors via EMT. Anticancer Res 35(2):689–696
Yates SC, Zafar A, Hubbard P, Nagy S, Durant S, Bicknell R, Wilcock G, Christie S, et al (2013) Dysfunction of the mTOR pathway is a risk factorfor Alzheimer’s disease. Acta Neuropathol Commun 1(3): doi:10.1186/2051-5960-1-3
Zeng LH, Rensing NR, Zhang B, Gutmann DH, Gambello MJ, Wong M (2011) Tsc2 gene inactivation causes a more severe epilepsy phenotype than Tsc1 inactivation in a mouse model of tuberous sclerosis complex. Hum Mol Genet 20(3):445–454. doi:10.1093/hmg/ddq491
Lim JS, Kim WI, Kang HC, Kim SH, Park AH, Park EK, Cho YW, Kim S et al (2015) Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy. Nat Med 21(4):395–400. doi:10.1038/nm.3824
Lee JH, Huynh M, Silhavy JL, Kim S, Dixon-Salazar T, Heiberg A, Scott E, Bafna V et al (2012) De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nat Genet 44(8):941–945. doi:10.1038/ng.2329
Zeng LH, Rensing NR, Wong M (2009) The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci: Off J Soc Neurosci 29(21):6964–6972. doi:10.1523/JNEUROSCI.0066-09.2009
Buckmaster PS, Ingram EA, Wen X (2009) Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci: Off J Soc Neurosci 29(25):8259–8269. doi:10.1523/JNEUROSCI.4179-08.2009
Huang X, Zhang H, Yang J, Wu J, McMahon J, Lin Y, Cao Z, Gruenthal M et al (2010) Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis 40(1):193–199. doi:10.1016/j.nbd.2010.05.024
Sha LZ, Xing XL, Zhang D, Yao Y, Dou WC, Jin LR, Wu LW, Xu Q (2012) Mapping the spatio-temporal pattern of the mammalian target of rapamycin (mTOR) activation in temporal lobe epilepsy. PLoS One 7(6):e39152. doi:10.1371/journal.pone.0039152
Sosunov AA, Wu X, McGovern RA, Coughlin DG, Mikell CB, Goodman RR, McKhann GM 2nd (2012) The mTOR pathway is activated in glial cells in mesial temporal sclerosis. Epilepsia 53(Suppl 1):78–86. doi:10.1111/j.1528-1167.2012.03478.x
Wang Y, Greenwood JS, Calcagnotto ME, Kirsch HE, Barbaro NM, Baraban SC (2007) Neocortical hyperexcitability in a human case of tuberous sclerosis complex and mice lacking neuronal expression of TSC1. Ann Neurol 61(2):139–152. doi:10.1002/ana.21058
Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, Jensen FE, Kwiatkowski DJ (2007) A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci: Off J Soc Neurosci 27(21):5546–5558. doi:10.1523/JNEUROSCI.5540-06.2007
Miralles VJ, Martinez-Lopez I, Zaragoza R, Borras E, Garcia C, Pallardo FV, Vina JR (2001) Na + dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) in primary astrocyte cultures: effect of oxidative stress. Brain Res 922(1):21–29. doi:10.1016/S0006-8993(01)03124-9
Sha L, Wu X, Yao Y, Wen B, Feng J, Sha Z, Wang X, Xing X et al (2014) Notch signaling activation promotes seizure activity in temporal lobe epilepsy. Mol Neurobiol 49(2):633–644. doi:10.1007/s12035-013-8545-0
Tauck DL, Nadler JV (1985) Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci: Off J Soc Neurosci 5(4):1016–1022
Frizzo MED, Lara DR, Prokopiuk AD, Vargas CR, Salbego CG, Wajner M, Souza DO (2002) Guanosine enhances glutamate uptake in brain cortical slices at normal and excitotoxic conditions. Cell Mol Neurobiol 22(3):353–363. doi:10.1023/A:1020728203682
Kriegstein A, Alvarez-Buylla A (2009) The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32:149–184. doi:10.1146/annurev.neuro.051508.135600
Williams PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, Dudek FE (2009) Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J Neurosci: Off J Soc Neurosci 29(7):2103–2112. doi:10.1523/JNEUROSCI.0980-08.2009
Codeluppi S, Svensson CI, Hefferan MP, Valencia F, Silldorff MD, Oshiro M, Marsala M, Pasquale EB (2009) The Rheb-mTOR pathway is upregulated in reactive astrocytes of the injured spinal cord. J Neurosci: Off J Soc Neurosci 29(4):1093–1104. doi:10.1523/JNEUROSCI.4103-08.2009
Zeng L-H, Xu L, Gutmann DH, Wong M (2008) Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol 63:444–453. doi:10.1002/ana.21331
Buckmaster PS, Lew FH (2011) Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci: Off J Soc Neurosci 31(6):2337–2347. doi:10.1523/JNEUROSCI.4852-10.2011
Heng K, Haney MM, Buckmaster PS (2013) High-dose rapamycin blocks mossy fiber sprouting but not seizures in a mouse model of temporal lobe epilepsy. Epilepsia 54(9):1535–1541. doi:10.1111/epi.12246
Robel S, Buckingham SC, Boni JL, Campbell SL, Danbolt NC, Riedemann T, Sutor B, Sontheimer H (2015) Reactive astrogliosis causes the development of spontaneous seizures. J Neurosci: Off J Soc Neurosci 35(8):3330–3345. doi:10.1523/JNEUROSCI.1574-14.2015
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65(1):1–105. doi:10.1016/S0301-0082(00)00067-8
Huang YH, Bergles DE (2004) Glutamate transporters bring competition to the synapse. Curr Opin Neurobiol 14(3):346–352. doi:10.1016/j.conb.2004.05.007
Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM, Bergles DE, Rothstein JD (2007) Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J Neurosci: Off J Soc Neurosci 27(25):6607–6619. doi:10.1523/JNEUROSCI.0790-07.2007
Petr GT, Sun Y, Frederick NM, Zhou Y, Dhamne SC, Hameed MQ, Miranda C, Bedoya EA et al (2015) Conditional deletion of the glutamate transporter GLT-1 reveals that astrocytic GLT-1 protects against fatal epilepsy while neuronal GLT-1 contributes significantly to glutamate uptake into synaptosomes. J Neurosci: Off J Soc Neurosci 35(13):5187–5201. doi:10.1523/JNEUROSCI.4255-14.2015
Sitcheran R, Gupta P, Fisher PB, Baldwin AS (2005) Positive and negative regulation of EAAT2 by NF-kappaB: a role for N-myc in TNFalpha-controlled repression. EMBO J 24(3):510–520. doi:10.1038/sj.emboj.7600555
Wu X, Kihara T, Akaike A, Niidome T, Sugimoto H (2010) PI3K/Akt/mTOR signaling regulates glutamate transporter 1 in astrocytes. Biochem Biophys Res Commun 393(3):514–518. doi:10.1016/j.bbrc.2010.02.038
Mayer SI, Rossler OG, Endo T, Charnay P, Thiel G (2009) Epidermal-growth-factor-induced proliferation of astrocytes requires Egr transcription factors. J Cell Sci 122(Pt 18):3340–3350. doi:10.1242/jcs.048272
Zelenaia OA, Robinson MB (2000) Degradation of glial glutamate transporter mRNAs is selectively blocked by inhibition of cellular transcription. J Neurochem 75(6):2252–2258. doi:10.1046/j.1471-4159.2000.0752252.x
Buckingham SC, Campbell SL, Haas BR, Montana V, Robel S, Ogunrinu T, Sontheimer H (2011) Glutamate release by primary brain tumors induces epileptic activity. Nat Med 17(10):1269–1274. doi:10.1038/nm.2453
Eid T, Tu N, Lee TS, Lai JC (2013) Regulation of astrocyte glutamine synthetase in epilepsy. Neurochem Int 63(7):670–681. doi:10.1016/j.neuint.2013.06.008
Sheldon AL, Gonzalez MI, Krizman-Genda EN, Susarla BT, Robinson MB (2008) Ubiquitination-mediated internalization and degradation of the astroglial glutamate transporter, GLT-1. Neurochem Int 53(6-8):296–308. doi:10.1016/j.neuint.2008.07.010
Martínez-Villarreal J, Tardón NG, Ibáñez I, Zafra CGF (2012) Cell surface turnover of the glutamate transporter GLT-1 is mediated by ubiquitination/deubiquitination. Glia 60:1356–1365. doi:10.1002/glia.22354
Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG (1994) Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci: Off J Soc Neurosci 14(9):5559–5569
Guo D, Zeng L, Brody DL, Wong M (2013) Rapamycin attenuates the development of posttraumatic epilepsy in a mouse model of traumatic brain injury. PLoS One 8(5):e64078. doi:10.1371/journal.pone.0064078
Chen L, Hu L, Dong JY, Ye Q, Hua N, Wong M, Zeng LH (2012) Rapamycin has paradoxical effects on S6 phosphorylation in rats with and without seizures. Epilepsia 53(11):2026–2033. doi:10.1111/epi.12013
Fedele DE, Gouder N, Guttinger M, Gabernet L, Scheurer L, Rulicke T, Crestani F, Boison D (2005) Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain: J Neurol 128(Pt 10):2383–2395. doi:10.1093/brain/awh555
During MJ, Spencer DD (1993) Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet 341(8861):1607–1610. doi:10.1016/0140-6736(93)90754-5
Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, Haydon PG, Coulter DA (2010) Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci 13(5):584–591. doi:10.1038/nn.2535
Goldshmit Y, Kanner S, Zacs M, Frisca F, Pinto AR, Currie PD, Pinkas-Kramarski R (2015) Rapamycin increases neuronal survival, reduces inflammation and astrocyte proliferation after spinal cord injury. Mol Cell Neurosci 68:82–91. doi:10.1016/j.mcn.2015.04.006
Banerjee S, Crouse NR, Emnett RJ, Gianino SM, Gutmann DH (2011) Neurofibromatosis-1 regulates mTOR-mediated astrocyte growth and glioma formation in a TSC/Rheb-independent manner. Proc Natl Acad Sci U S A 108(38):15996–16001. doi:10.1073/pnas.1019012108
Rothstein JD, Kammen MV, Levey AI, Kunc LJMRW (1995) Selective loss of glial glutamate transporter GLT‐1 in amyotrophic lateral sclerosis. Ann Neurol 38(1):73–84. doi:10.1002/ana.410380114
Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T et al (1997) Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276(5319):1699–1702. doi:10.1126/science.276.5319.1699
Jacob CP, Koutsilieri E, Bartl J, Neuen-Jacob E, Arzberger T, Zander N, Ravid R, Roggendorf W et al (2007) Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer's disease. J Alzheimers Dis: JAD 11(1):97–116
Li S, Mallory M, Alford M, Tanaka S, Masliah E (1997) Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J Neuropathol Exp Neurol 56(8):901–911. doi:10.1097/00005072-199708000-00008
Vanoni C, Massari S, Losa M, Carrega P, Perego C, Conforti L, Pietrini G (2004) Increased internalisation and degradation of GLT-1glial glutamate transporter in a cell model for familialamyotrophic lateral sclerosis (ALS). J Cell Sci 117(22):5417–5426. doi:10.1242/jcs
Cho CH (2011) Frontier of epilepsy research - mTOR signaling pathway. Exp Mol Med 43(5):231–274. doi:10.3858/emm.2011.43.5.032
Acknowledgments
We thank Prof. Weimin Tong, PUMC, for kindly providing genetic tools. We thank Dr. Susan Amara for providing CMV-hEAAT2 plasmid. This work was supported by the research grants from the National Basic Research Program of China (2013CB531301, 2012CB517902), the National Natural Science Foundation of China (31430048, 81471325, 31222031), the Fundamental Research Funds for the Central Universities (2012S05), and the PUMC Youth Fund (2012 J09, NCET-12-0071, 33320140172).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare no conflicts of interest.
Additional information
Xueqin Wang and Longze Sha contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1
(DOCX 153 kb)
Rights and permissions
About this article
Cite this article
Wang, X., Sha, L., Sun, N. et al. Deletion of mTOR in Reactive Astrocytes Suppresses Chronic Seizures in a Mouse Model of Temporal Lobe Epilepsy. Mol Neurobiol 54, 175–187 (2017). https://doi.org/10.1007/s12035-015-9590-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9590-7