Abstract
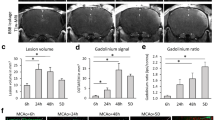
In view of its profound effect on cell survival and function, the modulation of the ubiquitin-proteasome-system has recently been shown to promote neurological recovery and brain remodeling after focal cerebral ischemia. Hitherto, local intracerebral delivery strategies were used, which can hardly be translated to human patients. We herein analyzed effects of systemic intraperitoneal delivery of the proteasome inhibitor BSc2118 on neurological recovery, brain injury, peripheral and cerebral immune responses, neurovascular integrity, as well as cerebral neurogenesis and angiogenesis in a mouse model of transient intraluminal middle cerebral artery occlusion. Systemic delivery of BSc2118 induced acute neuroprotection reflected by reduced infarct volume when delivered up to 9 h post-stroke. The latter was associated with reduced brain edema and stabilization of blood–brain–barrier integrity, albeit cerebral proteasome activity was only mildly reduced. Neuronal survival persisted in the post-acute stroke phase up to 28 days post-stroke and was associated with improved neurological recovery when the proteasome inhibitor was continuously delivered over 7 days. Systemic proteasome inhibition prevented stroke-induced acute leukocytosis in peripheral blood and reversed the subsequent immunosuppression, namely, the reduction of blood lymphocyte and granulocyte counts. On the contrary, post-ischemic brain inflammation, cerebral HIF-1α abundance, cell proliferation, neurogenesis, and angiogenesis were not influenced by the proteasome inhibitor. The modulation of peripheral immune responses might thus represent an attractive target for the clinical translation of proteasome inhibitors.







Similar content being viewed by others
References
Inobe T, Matouschek A (2014) Paradigms of protein degradation by the proteasome. Curr Opin Struct Biol 24:156–164. doi:10.1016/j.sbi.2014.02.002
Kniepert A, Groettrup M (2014) The unique functions of tissue-specific proteasomes. Trends Biochem Sci 39(1):17–24. doi:10.1016/j.tibs.2013.10.004
Buac D, Shen M, Schmitt S, Kona FR, Deshmukh R, Zhang Z, Neslund-Dudas C, Mitra B et al (2013) From bortezomib to other inhibitors of the proteasome and beyond. Curr Pharm Des 19(22):4025–4038
Xolalpa W, Perez-Galan P, Rodriguez MS, Roue G (2013) Targeting the ubiquitin proteasome system: beyond proteasome inhibition. Curr Pharm Des 19(22):4053–4093
Jankowska E, Stoj J, Karpowicz P, Osmulski PA, Gaczynska M (2013) The proteasome in health and disease. Curr Pharm Des 19(6):1010–1028
Elliott PJ, Ross JS (2001) The proteasome: a new target for novel drug therapies. Am J Clin Pathol 116(5):637–646. doi:10.1309/44HW-5YCJ-FLLP-3R56
Kukan M (2004) Emerging roles of proteasomes in ischemia-reperfusion injury of organs. J Physiol Pharmacol 55(1 Pt 1):3–15
Di Napoli M, McLaughlin B (2005) The ubiquitin-proteasome system as a drug target in cerebrovascular disease: therapeutic potential of proteasome inhibitors. Curr Opin Investig Drugs 6(7):686–699
Berti R, Williams AJ, Velarde LC, Moffett JR, Elliott PJ, Adams J, Yao C, Dave JR et al (2003) Effect of the proteasome inhibitor MLN519 on the expression of inflammatory molecules following middle cerebral artery occlusion and reperfusion in the rat. Neurotox Res 5(7):505–514
Buchan AM, Li H, Blackburn B (2000) Neuroprotection achieved with a novel proteasome inhibitor which blocks NF-kappaB activation. Neuroreport 11(2):427–430
Henninger N, Sicard KM, Bouley J, Fisher M, Stagliano NE (2006) The proteasome inhibitor VELCADE reduces infarction in rat models of focal cerebral ischemia. Neurosci Lett 398(3):300–305. doi:10.1016/j.neulet.2006.01.015
Phillips JB, Williams AJ, Adams J, Elliott PJ, Tortella FC (2000) Proteasome inhibitor PS519 reduces infarction and attenuates leukocyte infiltration in a rat model of focal cerebral ischemia. Stroke 31(7):1686–1693
Williams AJ, Hale SL, Moffett JR, Dave JR, Elliott PJ, Adams J, Tortella FC (2003) Delayed treatment with MLN519 reduces infarction and associated neurologic deficit caused by focal ischemic brain injury in rats via antiinflammatory mechanisms involving nuclear factor-kappaB activation, gliosis, and leukocyte infiltration. J Cereb Blood Flow Metab 23(1):75–87
Zhang L, Zhang ZG, Liu X, Hozeska A, Stagliano N, Riordan W, Lu M, Chopp M (2006) Treatment of embolic stroke in rats with bortezomib and recombinant human tissue plasminogen activator. Thromb Haemost 95(1):166–173
Zhang L, Zhang ZG, Zhang RL, Lu M, Adams J, Elliott PJ, Chopp M (2001) Postischemic (6-hour) treatment with recombinant human tissue plasminogen activator and proteasome inhibitor PS-519 reduces infarction in a rat model of embolic focal cerebral ischemia. Stroke 32(12):2926–2931
Zhang Y, Xiong M, Yan RQ, Sun FY (2010) Mutant ubiquitin-mediated beta-secretase stability via activation of caspase-3 is related to beta-amyloid accumulation in ischemic striatum in rats. J Cereb Blood Flow Metab 30(3):566–575. doi:10.1038/jcbfm.2009.228
Ruschak AM, Slassi M, Kay LE, Schimmer AD (2011) Novel proteasome inhibitors to overcome bortezomib resistance. J Natl Cancer Inst 103(13):1007–1017. doi:10.1093/jnci/djr160
Braun HA, Umbreen S, Groll M, Kuckelkorn U, Mlynarczuk I, Wigand ME, Drung I, Kloetzel PM et al (2005) Tripeptide mimetics inhibit the 20 S proteasome by covalent bonding to the active threonines. J Biol Chem 280(31):28394–28401. doi:10.1074/jbc.M502453200
Sterz J, Jakob C, Kuckelkorn U, Heider U, Mieth M, Kleeberg L, Kaiser M, Kloetzel PM et al (2010) BSc2118 is a novel proteasome inhibitor with activity against multiple myeloma. Eur J Haematol 85(2):99–107. doi:10.1111/j.1600-0609.2010.01450.x
Mlynarczuk-Bialy I, Doeppner TR, Golab J, Nowis D, Wilczynski GM, Parobczak K, Wigand ME, Hajdamowicz M et al (2014) Biodistribution and efficacy studies of the proteasome inhibitor BSc2118 in a mouse melanoma model. Transl Oncol 7(5):570–579. doi:10.1016/j.tranon.2014.07.002
Doeppner TR, Mlynarczuk-Bialy I, Kuckelkorn U, Kaltwasser B, Herz J, Hasan MR, Hermann DM, Bahr M (2012) The novel proteasome inhibitor BSc2118 protects against cerebral ischaemia through HIF1A accumulation and enhanced angioneurogenesis. Brain 135(Pt 11):3282–3297. doi:10.1093/brain/aws269
Doeppner TR, Kaltwasser B, Bahr M, Hermann DM (2014) Effects of neural progenitor cells on post-stroke neurological impairment—a detailed and comprehensive analysis of behavioral tests. Front Cell Neurosci 8:338. doi:10.3389/fncel.2014.00338
Chiba Y, Sasayama T, Miyake S, Koyama J, Kondoh T, Hosoda K, Kohmura E (2008) Anti-VEGF receptor antagonist (VGA1155) reduces infarction in rat permanent focal brain ischemia. Kobe J Med Sci 54(2):E136–146
Doeppner TR, Kaltwasser B, ElAli A, Zechariah A, Hermann DM, Bahr M (2011) Acute hepatocyte growth factor treatment induces long-term neuroprotection and stroke recovery via mechanisms involving neural precursor cell proliferation and differentiation. J Cereb Blood Flow Metab 31(5):1251–1262. doi:10.1038/jcbfm.2010.211
Doeppner TR, Kaltwasser B, Teli MK, Bretschneider E, Bahr M, Hermann DM (2014) Effects of acute versus post-acute systemic delivery of neural progenitor cells on neurological recovery and brain remodeling after focal cerebral ischemia in mice. Cell Death Dis 5, e1386. doi:10.1038/cddis.2014.359
Herz J, Hagen SI, Bergmuller E, Sabellek P, Gothert JR, Buer J, Hansen W, Hermann DM et al (2014) Exacerbation of ischemic brain injury in hypercholesterolemic mice is associated with pronounced changes in peripheral and cerebral immune responses. Neurobiol Dis 62:456–468. doi:10.1016/j.nbd.2013.10.022
Candelario-Jalil E, Yang Y, Rosenberg GA (2009) Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience 158(3):983–994. doi:10.1016/j.neuroscience.2008.06.025
Macrez R, Ali C, Toutirais O, Le Mauff B, Defer G, Dirnagl U, Vivien D (2011) Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol 10(5):471–480. doi:10.1016/S1474-4422(11)70066-7
Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E et al (2009) Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40(5):1849–1857. doi:10.1161/STROKEAHA.108.534503
Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, Ziemssen T, Prass K, Meisel A (2007) Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke 38(2 Suppl):770–773. doi:10.1161/01.STR.0000251441.89665.bc
Famakin BM (2014) The immune response to acute focal cerebral ischemia and associated post-stroke immunodepression: a focused review. Aging Dis 5(5):307–326. doi:10.14336/AD.2014.0500307
Ge P, Luo Y, Liu CL, Hu B (2007) Protein aggregation and proteasome dysfunction after brain ischemia. Stroke 38(12):3230–3236. doi:10.1161/STROKEAHA.107.487108
Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD (2006) Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab 26(5):654–665. doi:10.1038/sj.jcbfm.9600217
Dahlmann B (2007) Role of proteasomes in disease. BMC Biochem 8(Suppl 1):S3. doi:10.1186/1471-2091-8-S1-S3
Sinn DI, Lee ST, Chu K, Jung KH, Kim EH, Kim JM, Park DK, Song EC et al (2007) Proteasomal inhibition in intracerebral hemorrhage: neuroprotective and anti-inflammatory effects of bortezomib. Neurosci Res 58(1):12–18. doi:10.1016/j.neures.2007.01.006
Cunningham LA, Candelario K, Li L (2012) Roles for HIF-1alpha in neural stem cell function and the regenerative response to stroke. Behav Brain Res 227(2):410–417. doi:10.1016/j.bbr.2011.08.002
Mazumdar J, O’Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, Simon MC (2010) O2 regulates stem cells through Wnt/beta-catenin signalling. Nat Cell Biol 12(10):1007–1013. doi:10.1038/ncb2102
Tsai YW, Yang YR, Wang PS, Wang RY (2011) Intermittent hypoxia after transient focal ischemia induces hippocampal neurogenesis and c-Fos expression and reverses spatial memory deficits in rats. PLoS ONE 6(8), e24001. doi:10.1371/journal.pone.0024001
Shi H (2009) Hypoxia inducible factor 1 as a therapeutic target in ischemic stroke. Curr Med Chem 16(34):4593–4600
Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002) Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8(9):963–970
Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, Lazarov O (2014) Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here. J Cereb Blood Flow Metab 34(10):1573–1584. doi:10.1038/jcbfm.2014.130
Hermann DM, Chopp M (2012) Promoting brain remodelling and plasticity for stroke recovery: therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol 11(4):369–380. doi:10.1016/S1474-4422(12)70039-X
Xiong Y, Mahmood A, Chopp M (2010) Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs 11(3):298–308
Yu TS, Washington PM, Kernie SG (2014) Injury-induced neurogenesis: mechanisms and relevance. Neuroscientist. doi:10.1177/1073858414563616
Acknowledgments
This study was supported by grants from the German Research Council (DFG, No. HE3173/2-2 and No. HE3173/3-1 to DMH) and a grant from the Scientific and Technological Research Council of Turkey (TUBITAK, No. 2221 to TRD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All studies were performed according to local government authorities.
Conflict of Interest
The authors declare that they have no competing interests.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 35 kb)
Rights and permissions
About this article
Cite this article
Doeppner, T.R., Kaltwasser, B., Kuckelkorn, U. et al. Systemic Proteasome Inhibition Induces Sustained Post-stroke Neurological Recovery and Neuroprotection via Mechanisms Involving Reversal of Peripheral Immunosuppression and Preservation of Blood–Brain–Barrier Integrity. Mol Neurobiol 53, 6332–6341 (2016). https://doi.org/10.1007/s12035-015-9533-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9533-3