Abstract
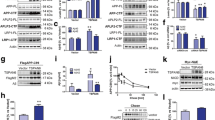
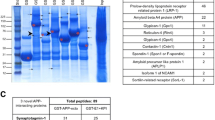
Maintenance of intracellular proteostasis is essential for neuronal function, and emerging data support the view that disturbed proteostasis plays an important role in brain aging and the pathogenesis of age-related neurodegenerative disorders such as Alzheimer’s disease (AD). sAPPalpha (sAPPα), the extracellularly secreted N-terminal alpha secretase cleavage product of the amyloid precursor protein (APP), has an established function in neuroprotection. Recently, we provided evidence that membrane-bound holo-APP functionally cooperates with sAPPα to mediate neuroprotection via activation of the Akt survival signaling pathway and sAPPα directly affects proteostasis. Here, we demonstrate that in addition to its anti-apoptotic function, sAPPα has effects on neuronal proteostasis under conditions of proteasomal stress. In particular, recombinant sAPPα significantly suppressed MG132-triggered expression of the co-chaperone BAG3 and aggresome formation, and it partially rescued proteasomal activity in a dose-dependent manner in SH-SY5Y neuroblastoma cells. In analogy, sAPPα was able to inhibit MG132-induced BAG3 expression in primary hippocampal neurons. Strikingly, these sAPPα-induced changes were unaltered in APP-depleted SH-SY5Y cells and APP-deficient neurons, demonstrating that holo-APP is not required for this particular function of sAPPα. Importantly, recombinant sAPPbeta (sAPPβ) failed to modulate BAG3 expression and proteostasis in APP-proficient wild-type (wt) cells, indicating that these biological effects are highly selective for sAPPα. In conclusion, we demonstrate that modulation of proteostasis is a distinct biological function of sAPPα and does not require surface-bound holo-APP. Our data shed new light on the physiological functions of APP and the interplay between APP processing and proteostasis during brain aging.




Similar content being viewed by others
References
Nhan HS, Chiang K, Koo EH (2015) The multifaceted nature of amyloid precursor protein and its proteolytic fragments: friends and foes. Acta Neuropathol 129(1):1–19. doi:10.1007/s00401-014-1347-2
Kögel D, Deller T, Behl C (2012) Roles of amyloid precursor protein family members in neuroprotection, stress signaling and aging. Exp Brain Res Experimentelle Hirnforschung 217(3-4):471–479
Copanaki E, Chang S, Vlachos A, Tschape JA, Muller UC, Kogel D, Deller T (2010) sAPPalpha antagonizes dendritic degeneration and neuron death triggered by proteasomal stress. Mol Cell Neurosci 44(4):386–393
Eckert GP, Chang S, Eckmann J, Copanaki E, Hagl S, Hener U, Muller WE, Kogel D (2011) Liposome-incorporated DHA increases neuronal survival by enhancing non-amyloidogenic APP processing. Biochim Biophys Acta 1808(1):236–243
Kögel D, Schomburg R, Copanaki E, Prehn JH (2005) Regulation of gene expression by the amyloid precursor protein: inhibition of the JNK/c-Jun pathway. Cell Death Differ 12(1):1–9
Milosch N, Tanriover G, Kundu A, Rami A, Francois JC, Baumkotter F, Weyer SW, Samanta A et al (2014) Holo-APP and G-protein-mediated signaling are required for sAPPalpha-induced activation of the Akt survival pathway. Cell Death Dis 5:e1391. doi:10.1038/cddis.2014.352
Gralle M, Botelho MG, Wouters FS (2009) Neuroprotective secreted amyloid precursor protein acts by disrupting amyloid precursor protein dimers. J Biol Chem 284(22):15016–15025. doi:10.1074/jbc.M808755200
Renziehausen J, Hiebel C, Nagel H, Kundu A, Kins S, Kogel D, Behl C, Hajieva P (2015) The cleavage product of amyloid-beta protein precursor sAbetaPPalpha modulates BAG3-dependent aggresome formation and enhances cellular proteasomal activity. J Alzheimers Dis 44(3):879–896. doi:10.3233/JAD-140600
Mohrenz IV, Antonietti P, Pusch S, Capper D, Balss J, Voigt S, Weissert S, Mukrowsky A et al (2013) Isocitrate dehydrogenase 1 mutant R132H sensitizes glioma cells to BCNU-induced oxidative stress and cell death. Apoptosis 18(11):1416–1425. doi:10.1007/s10495-013-0877-8
Cappai R, Mok SS, Galatis D, Tucker DF, Henry A, Beyreuther K, Small DH, Masters CL (1999) Recombinant human amyloid precursor-like protein 2 (APLP2) expressed in the yeast Pichia pastoris can stimulate neurite outgrowth. FEBS Lett 442(1):95–98
Henry A, Masters CL, Beyreuther K, Cappai R (1997) Expression of human amyloid precursor protein ectodomains in Pichia pastoris: analysis of culture conditions, purification, and characterization. Protein Expr Purif 10(2):283–291. doi:10.1006/prep.1997.0748
Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RL, Hess S, Chan DC (2011) Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet 20(9):1726–1737. doi:10.1093/hmg/ddr048
Behl C (2011) BAG3 and friends: co-chaperones in selective autophagy during aging and disease. Autophagy 7(7):795–798. doi:10.4161/auto.7.7.15844
Gamerdinger M, Kaya AM, Wolfrum U, Clement AM, Behl C (2011) BAG3 mediates chaperone-based aggresome-targeting and selective autophagy of misfolded proteins. EMBO Rep 12(2):149–156. doi:10.1038/embor.2010.203
Kern A, Roempp B, Prager K, Walter J, Behl C (2006) Down-regulation of endogenous amyloid precursor protein processing due to cellular aging. J Biol Chem 281(5):2405–2413
Selkoe DJ (2011) Alzheimer’s disease. Cold Spring Harb Perspect Biol 3 (7). doi:10.1101/cshperspect.a004457
Soba P, Eggert S, Wagner K, Zentgraf H, Siehl K, Kreger S, Lower A, Langer A et al (2005) Homo- and heterodimerization of APP family members promotes intercellular adhesion. EMBO J 24(20):3624–3634
Corrigan F, Pham CL, Vink R, Blumbergs PC, Masters CL, van den Heuvel C, Cappai R (2011) The neuroprotective domains of the amyloid precursor protein, in traumatic brain injury, are located in the two growth factor domains. Brain Res 1378:137–143. doi:10.1016/j.brainres.2010.12.077
Gersbacher MT, Goodger ZV, Trutzel A, Bundschuh D, Nitsch RM, Konietzko U (2013) Turnover of amyloid precursor protein family members determines their nuclear signaling capability. PLoS One 8(7):e69363. doi:10.1371/journal.pone.0069363
Morawe T, Hiebel C, Kern A, Behl C (2012) Protein homeostasis, aging and Alzheimer’s disease. Mol Neurobiol 46(1):41–54
Tanaka K, Matsuda N (2014) Proteostasis and neurodegeneration: the roles of proteasomal degradation and autophagy. Biochim Biophys Acta 1843(1):197–204. doi:10.1016/j.bbamcr.2013.03.012
Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU, Behl C (2009) Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J 28(7):889–901
Endres K, Fahrenholz F (2012) Regulation of alpha-secretase ADAM10 expression and activity. Exp Brain Res 217(3-4):343–352. doi:10.1007/s00221-011-2885-7
Furukawa K, Sopher BL, Rydel RE, Begley JG, Pham DG, Martin GM, Fox M, Mattson MP (1996) Increased activity-regulating and neuroprotective efficacy of alpha-secretase-derived secreted amyloid precursor protein conferred by a C-terminal heparin-binding domain. J Neurochem 67(5):1882–1896
Hick M, Herrmann U, Weyer SW, Mallm JP, Tschape JA, Borgers M, Mercken M, Roth FC et al (2015) Acute function of secreted amyloid precursor protein fragment APPsalpha in synaptic plasticity. Acta Neuropathol 129(1):21–37. doi:10.1007/s00401-014-1368-x
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (DFG, grants KO 1898/6-1 and 10-1 to DK; BE 1475/8-1 to CB; KI 819/5-1 and 819/6-1 to SK; MU 1457/8-1 and 1457/9-1 to UCM; and DFG/Collaborative Research Center 1080 to CB). We thank Gabriele Köpf for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Kundu, A., Milosch, N., Antonietti, P. et al. Modulation of BAG3 Expression and Proteasomal Activity by sAPPα Does Not Require Membrane-Tethered Holo-APP. Mol Neurobiol 53, 5985–5994 (2016). https://doi.org/10.1007/s12035-015-9501-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9501-y