Abstract
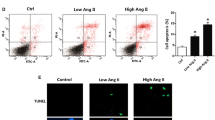
Our recent study indicated that angiotensin II (Ang II), the main component of renin-angiotensin system, participated in the pathogenesis of Parkinson’s disease (PD) by triggering the apoptosis of dopaminergic neuronal cells. However, the underlying mechanisms are still not fully understood. In this study, by using CATH.a cells, a dopaminergic neuronal cell line stably expressing angiotensin II type 1 receptor (AT1R) and angiotensin II type 2 receptor (AT2R), we tested the hypothesis that activation of autophagy contributed to the apoptosis triggered by Ang II. We showed that Ang II activated autophagy and triggered apoptosis in CATH.a cells in a dose-dependent manner. More importantly, inhibition of autophagy by 3-methyladenine markedly attenuated the apoptosis caused by Ang II in CATH.a cells. In addition, the Ang II-induced autophagy and subsequent cell apoptosis could be fully abolished by an AT1R antagonist losartan rather than PD1223319, an antagonist for AT2R. Taken together, our study provides the first evidence that Ang II triggers apoptosis via activation of autophagy in a dopaminergic neuronal cell line through an AT1R-mediated manner. These findings have deepened our understanding on the role of Ang II in the pathogenesis of PD and support the use of AT1R antagonists for the treatment of this devastating neurodegenerative disease.





Similar content being viewed by others
References
Ulrich JD, Finn MB, Wang Y, Shen A, Mahan TE, Jiang H, Stewart FR, Piccio L et al (2014) Altered microglial response to Abeta plaques in APPPS1-21 mice heterozygous for TREM2. Mol Neurodegener 9:20. doi:10.1186/1750-1326-9-20
Jiang T, Yu JT, Tian Y, Tan L (2013) Epidemiology and etiology of Alzheimer's disease: from genetic to non-genetic factors. Curr Alzheimer Res 10(8):852–867
Schapira AH (2009) Neurobiology and treatment of Parkinson's disease. Trends Pharmacol Sci 30(1):41–47. doi:10.1016/j.tips.2008.10.005
Schapira AH, Jenner P (2011) Etiology and pathogenesis of Parkinson's disease. Mov Disord 26(6):1049–1055. doi:10.1002/mds.23732
Nguyen Dinh Cat A, Touyz RM (2011) A new look at the renin-angiotensin system—focusing on the vascular system. Peptides 32(10):2141–2150. doi:10.1016/j.peptides.2011.09.010
Herr D, Bekes I, Wulff C (2013) Local renin-angiotensin system in the reproductive system. Front Endocrinol 4:150. doi:10.3389/fendo.2013.00150
Marquez E, Riera M, Pascual J, Soler MJ (2014) Renin angiotensin system within the diabetic podocyte. Am J Physiol Renal Physiol 00531:02013. doi:10.1152/ajprenal.00531.2013
Giese MJ, Speth RC (2014) The ocular renin-angiotensin system: a therapeutic target for the treatment of ocular disease. Pharmacol Ther 142(1):11–32. doi:10.1016/j.pharmthera.2013.11.002
Wright JW, Harding JW (2011) Brain renin-angiotensin—a new look at an old system. Prog Neurobiol 95(1):49–67. doi:10.1016/j.pneurobio.2011.07.001
Jiang T, Gao L, Lu J, Zhang YD (2013) ACE2-Ang-(1-7)-Mas axis in brain: a potential target for prevention and treatment of ischemic stroke. Curr Neuropharmacol 11(2):209–217. doi:10.2174/1570159X11311020007
Labandeira-Garcia JL, Garrido-Gil P, Rodriguez-Pallares J, Valenzuela R, Borrajo A, Rodriguez-Perez AI (2014) Brain renin-angiotensin system and dopaminergic cell vulnerability. Front Neuroanat 8:67. doi:10.3389/fnana.2014.00067
Zhao HR, Jiang T, Tian YY, Gao Q, Zhang L, Pan Y, Wu L, Lu J et al (2015) Angiotensin II Triggers Apoptosis Via Enhancement of NADPH Oxidase-dependent oxidative stress in a dopaminergic neuronal cell line. Neurochem Res 40(4):854–863. doi:10.1007/s11064-015-1536-y
Wu L, Tian YY, Shi JP, Xie W, Shi JQ, Lu J, Zhang YD (2013) Inhibition of endoplasmic reticulum stress is involved in the neuroprotective effects of candesartan cilexitil in the rotenone rat model of Parkinson's disease. Neurosci Lett 548:50–55. doi:10.1016/j.neulet.2013.06.008
Sonsalla PK, Coleman C, Wong LY, Harris SL, Richardson JR, Gadad BS, Li W, German DC (2013) The angiotensin converting enzyme inhibitor captopril protects nigrostriatal dopamine neurons in animal models of parkinsonism. Exp Neurol 250:376–383. doi:10.1016/j.expneurol.2013.10.014
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290(5497):1717–1721
Shintani T, Klionsky DJ (2004) Autophagy in health and disease: a double-edged sword. Science 306(5698):990–995. doi:10.1126/science.1099993
Martinet W, Agostinis P, Vanhoecke B, Dewaele M, De Meyer GR (2009) Autophagy in disease: a double-edged sword with therapeutic potential. Clin Sci (Lond) 116(9):697–712. doi:10.1042/CS20080508
Lu J, Wu L, Jiang T, Wang Y, Zhao H, Gao Q, Pan Y, Tian Y et al (2014) Angiotensin AT receptor stimulation inhibits activation of NADPH oxidase and ameliorates oxidative stress in rotenone model of Parkinson's disease in CATH.a cells. Neurotoxicol Teratol 47C:16–24. doi:10.1016/j.ntt.2014.11.004
Jiang T, Yu JT, Zhu XC, Tan MS, Wang HF, Cao L, Zhang QQ, Shi JQ et al (2014) Temsirolimus promotes autophagic clearance of amyloid-beta and provides protective effects in cellular and animal models of Alzheimer's disease. Pharmacol Res 81:54–63. doi:10.1016/j.phrs.2014.02.008
Jiang T, Yu JT, Zhu XC, Zhang QQ, Tan MS, Cao L, Wang HF, Shi JQ et al (2014) Ischemic preconditioning provides neuroprotection by induction of AMP-activated protein kinase-dependent autophagy in a rat model of ischemic stroke. Mol Neurobiol. doi:10.1007/s12035-014-8725-6
Zhu XC, Jiang T, Zhang QQ, Cao L, Tan MS, Wang HF, Ding ZZ, Tan L et al (2014) Chronic metformin preconditioning provides neuroprotection via suppression of NF-kappaB-mediated inflammatory pathway in rats with permanent cerebral ischemia. Mol Neurobiol. doi:10.1007/s12035-014-8866-7
Wu L, Luo N, Zhao HR, Gao Q, Lu J, Pan Y, Shi JP, Tian YY et al (2014) Salubrinal protects against rotenone-induced SH-SY5Y cell death via ATF4-parkin pathway. Brain Res 1549:52–62. doi:10.1016/j.brainres.2014.01.003
Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, Zhang QQ, Gao L et al (2014) Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol 171(13):3146–3157. doi:10.1111/bph.12655
Jiang T, Yu JT, Zhu XC, Zhang QQ, Tan MS, Cao L, Wang HF, Lu J et al (2014) Angiotensin-(1-7) induces cerebral ischemic tolerance by promoting brain angiogenesis in a Mas/eNOS-dependent pathway. Br J Pharmacol. doi:10.1111/bph.12770
Jiang T, Gao L, Zhu XC, Yu JT, Shi JQ, Tan MS, Lu J, Tan L et al (2013) Angiotensin-(1-7) inhibits autophagy in the brain of spontaneously hypertensive rats. Pharmacol Res 71:61–68. doi:10.1016/j.phrs.2013.03.001
Creagh EM (2014) Caspase crosstalk: integration of apoptotic and innate immune signalling pathways. Trends Immunol 35(12):631–640. doi:10.1016/j.it.2014.10.004
Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E (2005) Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy 1(2):84–91
Jaakkola PM, Pursiheimo JP (2009) p62 degradation by autophagy: another way for cancer cells to survive under hypoxia. Autophagy 5(3):410–412
Yadav A, Vallabu S, Arora S, Tandon P, Slahan D, Teichberg S, Singhal PC (2010) ANG II promotes autophagy in podocytes. Am J Physiol Cell Physiol 299(2):C488–C496. doi:10.1152/ajpcell.00424.2009
Porrello ER, D'Amore A, Curl CL, Allen AM, Harrap SB, Thomas WG, Delbridge LM (2009) Angiotensin II type 2 receptor antagonizes angiotensin II type 1 receptor-mediated cardiomyocyte autophagy. Hypertension 53(6):1032–1040. doi:10.1161/HYPERTENSIONAHA.108.128488
Yu KY, Wang YP, Wang LH, Jian Y, Zhao XD, Chen JW, Murao K, Zhu W et al (2014) Mitochondrial KATP channel involvement in angiotensin II-induced autophagy in vascular smooth muscle cells. Basic Res Cardiol 109(4):416. doi:10.1007/s00395-014-0416-y
Zhu XC, Yu JT, Jiang T, Tan L (2013) Autophagy modulation for Alzheimer's disease therapy. Mol Neurobiol 48(3):702–714. doi:10.1007/s12035-013-8457-z
Hu Z, Yang B, Mo X, Xiao H (2014) Mechanism and regulation of autophagy and its role in neuronal diseases. Mol Neurobiol. doi:10.1007/s12035-014-8921-4
Smith CM, Chen Y, Sullivan ML, Kochanek PM, Clark RS (2011) Autophagy in acute brain injury: feast, famine, or folly? Neurobiol Dis 43(1):52–59. doi:10.1016/j.nbd.2010.09.014
Chu CT (2008) Eaten alive: autophagy and neuronal cell death after hypoxia-ischemia. Am J Pathol 172(2):284–287. doi:10.2353/ajpath.2008.071064
Xu J, Qin X, Cai X, Yang L, Xing Y, Li J, Zhang L, Tang Y et al (2015) Mitochondrial JNK activation triggers autophagy and apoptosis and aggravates myocardial injury following ischemia/reperfusion. Biochim Biophys Acta 1852(2):262–270. doi:10.1016/j.bbadis.2014.05.012
Wang JY, Xia Q, Chu KT, Pan J, Sun LN, Zeng B, Zhu YJ, Wang Q et al (2011) Severe global cerebral ischemia-induced programmed necrosis of hippocampal CA1 neurons in rat is prevented by 3-methyladenine: a widely used inhibitor of autophagy. J Neuropathol Exp Neurol 70(4):314–322. doi:10.1097/NEN.0b013e31821352bd
Mishra AK, Ur Rasheed MS, Shukla S, Tripathi MK, Dixit A, Singh MP (2014) Aberrant autophagy and parkinsonism: does correction rescue from disease progression? Mol Neurobiol. doi:10.1007/s12035-014-8744-3
Wang X, Dai Y, Ding Z, Khaidakov M, Mercanti F, Mehta JL (2013) Regulation of autophagy and apoptosis in response to angiotensin II in HL-1 cardiomyocytes. Biochem Biophys Res Commun 440(4):696–700. doi:10.1016/j.bbrc.2013.09.131
Funding
This work was supported by the grants from the Innovation Project for Postgraduates of Jiangsu Province to T.J. (CXLX13_561), the National Natural Science Foundation of China (81271418) and the Six Talent Summit of Jiangsu Province to Y.D.Z. (N02012-WS-086), and the Natural Science Foundation of Jiangsu Province to Y.Y.T. (BK2012524).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Qing Gao and Teng Jiang should be regarded as co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Effects of 3-MA, losartan and PD123319 on apoptosis and basal activity of autophagy in CATH.a cells. CATH.a cells were treated with 3-MA (5 mM), losartan (1μM) or PD123319 (1μM) for 24 hours, and (A) the protein level of LC3-II was evaluated by Western blot, β-actin was used as loading control. (B) The activity of caspase-3 was directly measured by a colorimetric assay kit. All figures are representative of three independent experiments, performed in triplicate. Data were analyzed by one-way ANOVA followed by Tukey’s post hoc test. Columns represent mean±s.e.m. *P<0.05 vs. control group. (TIFF 283 kb)
Rights and permissions
About this article
Cite this article
Gao, Q., Jiang, T., Zhao, HR. et al. Activation of Autophagy Contributes to the Angiotensin II-Triggered Apoptosis in a Dopaminergic Neuronal Cell Line. Mol Neurobiol 53, 2911–2919 (2016). https://doi.org/10.1007/s12035-015-9177-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9177-3