Abstract
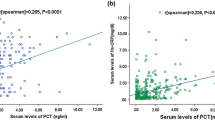
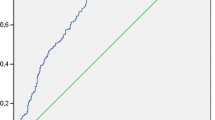
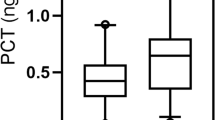
Inflammatory markers have been associated with functional outcome and mortality of stroke. We investigated the changes in procalcitonin (PCT) and high-sensitivity C-reactive protein (Hs-CRP) levels during the acute period of ischemic stroke and evaluated the relationship between these levels and the long-term functional outcome and mortality. We prospectively studied 376 patients with acute ischemic stroke (AIS) who were admitted within 24 h after the onset of symptoms. PCT, Hs-CRP, and NIH Stroke Scale (NIHSS) were measured at the time of admission. Long-term functional outcome were measured by modified Rankin scale (mRS) at 1 year after admission. The correlations between the levels of PCT, Hs-CRP, and mortality at 1 year after stroke onset were analyzed. Patients with poor with functional outcome and non-survivors had significantly increased PCT and Hs-CRP levels on admission. Multivariate logistic regression analysis showed that PCT was an independent prognostic marker of 1-year functional outcome and death [odds ratio (OR) 2.33 (95 % CI, 1.33–3.44) and 3.11 (2.02–4.43), respectively, P < 0.0001 for both, adjusted for age, NIHSS, other predictors, and vascular risk factors] in patients with AIS. The area under the receiver operating characteristic curve of PCT was 0.77 (95 % CI, 0.72–0.83) for functional outcome and 0.88 (95 % CI, 0.84–0.93) for mortality. PCT improved the area under the receiver operating characteristic curve of the NIHSS score for functional outcome from 0.74 (95 % CI, 0.66–0.81) to 0.85 (95 % CI, 0.76–0.92; P < 0.0001) and for mortality from 0.77 (95 % CI, 0.70–0.83) to 0.94 (95 % CI, 0.89–0.97; P < 0.0001). Serum level of PCT at admission was an independent predictor of long-term functional outcome and mortality after ischemic stroke in Chinese sample.



Similar content being viewed by others
References
Zhang ZG, Wang C, Wang J, Zhang Z, Yang YL, Gao L, Zhang XY, Chang T et al (2014) Prognostic value of mannose-binding lectin: 90-day outcome in patients with acute ischemic stroke. Mol Neurobiol doi:10.1007/s12035-014-8682-0
Slot KB, Berge E, Dorman P, Lewis S, Dennis M, Sandercock P (2008) Impact of functional status at six months on long term survival in patients with ischaemic stroke: prospective cohort studies. BMJ 336:376–379
Tu WJ, Dong X, Zhao SJ, Yang DG, Chen H (2013) Prognostic value of plasma neuroendocrine biomarkers in patients with acute ischaemic stroke. J Neuroendocrinol 25:771–778
Tu WJ, Zhao SJ, Liu TG et al (2013) Combination of high-sensitivity C-reactive protein and homocysteine predicts the short-term outcomes of Chinese patients with acute ischemic stroke. Neurol Res 35(9):912–921
Vibo R, Kõrv J, Roose M, Kampus P, Muda P, Zilmer K, Zilmer M (2007) Free Radic Res. Acute phase proteins and oxidised low-density lipoprotein in association with ischemic stroke subtype, severity and outcome. Free Radic Res 41(3):282–287
Yoldas T, Gonen M, Godekmerdan A, Ilhan F, Bayram E (2007) The serum high-sensitive C reactive protein and homocysteine levels to evaluate the prognosis of acute ischemic stroke. Mediators Inflamm 2007:15929
Shantikumar S, Grant PJ, Catto AJ, Bamford JM, Carter AM (2009) Elevated C-reactive protein and long-term mortality after ischaemic stroke: Relationship with markers of endothelial cell and platelet activation. Stroke 40:977–979
Di Napoli M, Godoy DA, Campi V et al (2011) C-reactive protein level measurement improves mortality prediction when added to the spontaneous intracerebral hemorrhage score. Stroke 42:1230–1236
Huang Y, Jing J, Zhao XQ, Wang CX, Wang YL, Liu GF, Wang CJ, Liu LP et al (2012) High-sensitivity C-reactive protein is a strong risk factor for death after acute ischemic stroke among Chinese. CNS Neurosci Ther 18(3):261–266
Maruna P, Nedelnikova K, Gurlich R (2000) Physiology and genetics of procalcitonin. Physiol Res 49:S57–S62
Sakr Y, Sponholz C, Tuche F, Brunkhorst F, Reinhart K (2008) The role of procalcitonin in febrile neutropenic patients: review of the literature. Infection 36:396–407
McGrane S, Girard TD, Thompson JL et al (2011) Procalcitonin and C-reactive protein levels at admission as predictors of duration of acute brain dysfunction in critically ill patients. Crit Care 15(2):R78
Mimoz O, Edouard AR, Samii K et al (1998) Procalcitonin and C-reactive protein during the early posttraumatic systemic inflammatory response syndrome. Intensive Care Med 24(2):185–188
Schiopu A, Hedblad B, Engström G et al (2012) Plasma procalcitonin and the risk of cardiovascular events and death: a prospective population‐based study. J Intern Med 272(5):484–491
Katan M, Moon YP, DeRosa J et al (2014) Procalcitonin, copeptin and midregional pro-atrial natriuretic peptide as markers of ischemic stroke risk: the Northern Manhattan Study. Stroke 45(Suppl 1):A54–A54
Hatano S (1976) Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ 54:541–553
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24:35–41
Bamford J, Sandercock P, Dennis M et al (1991) Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 337:1521–1526
Brott T, Marler JR, Olinger CP, Adams HP Jr, Tomsick T, Barsan WG et al (1989) Measurements of acute cerebral infarction: lesion size by computed tomography. Stroke 20:871–875
Zhang W, Zhang XA (2014) Prognostic value of serum lipoprotein(a) levels in patients with acute ischemic stroke. Neuroreport 25(4):262–266
Bonita RBR (1988) Modification of Rankin Scale: recovery of motor function after stroke. Stroke 19:1497–1500
Simon L, Gauvin F, Amre DK et al (2004) Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis 39(2):206–217
Danesh J, Collins R, Appleby P, Peto R (1998) Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 279:1477–1482
Elkind MS, Tai W, Coates K, Paik MC, Sacco RL (2006) High-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, and outcome after ischemic stroke. Arch Intern Med 166(19):2073–2080
Tamaki K, Kogata Y, Sugiyama D, Nakazawa T, Hatachi S, Kageyama G, Nishimura K, Morinobu A et al (2008) Diagnostic accuracy of serum procalcitonin concentrations for detecting systemic bacterial infection in patients with systemic autoimmune diseases. J Rheumatol 35(1):114–119
Schuetz P, Suter-Widmer I, Chaudri A, Christ-Crain M, Zimmerli W et al (2011) Prognostic value of procalcitonin in community-acquired pneumonia. Eur Respir J 37:384–392
Luyt CE, Guérin V, Combes A et al (2005) Procalcitonin kinetics as a prognostic marker of ventilator-associated pneumonia. Am J Respir Crit Care Med 171(1):48–53
Fluri F, Morgenthaler NG, Mueller B, Christ-Crain M, Katan M (2012) Copeptin, Procalcitonin and routine inflammatory markers—predictors of infection after stroke. PLoS One 7(10):e48309
Sacco RL, Boden-Albala B, Chen X, Lin IF, Kargman DE, Paik MC (1998) Relationship of 6-month functional outcome and stroke severity: implications for acute stroke trials from the Northern Manhattan Stroke Study [abstract]. Neurology 50(suppl 4):A327
Adams HP Jr, Davis PH, Leira EC et al (1999) Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 53:126–131
Castelli GP, Pognani C, Meisner M et al (2004) Procalcitonin and C-reactive protein during systemic inflammatory response syndrome, sepsis and organ dysfunction. Crit Care 8(4):R234
Wheeler AP, Bernard GR (1999) Treating patients with severe sepsis. N Engl J Med 340:207–214
Acknowledgments
We also express our gratitude to all the patients who participated in this study and thereby made this work possible.
Authors’ Contributions
Li-Hong Li had full access to all the data in the study and is responsible for the integrity of the data and the accuracy of the data analyses. Chao Wang, Li Gao, and Zhi-Guo Zhang contributed in the study concept and design and drafting of the manuscript. Zhi-Guo Zhang, Yu-Qian Li, Yan-Long Yang, and Tao-Chang Zheng contributed in the acquisition of data. J Chao Wang, Li Gao, Zhi-Guo Zhang, Long-Long Zheng, Xing-Ye Zhang, and Ming-Hao Man contributed in the analysis and interpretation of data. Li-Hong Li contributed in the critical revision of the manuscript for important intellectual content, and administrative support. All authors have read, edited, and approved the final version of the manuscript.
Conflict of Interest
The authors have no relevant potential conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Chao Wang, Li Gao, and Zhi-Guo Zhang contributed equally to this study.
Rights and permissions
About this article
Cite this article
Wang, C., Gao, L., Zhang, ZG. et al. Procalcitonin Is a Stronger Predictor of Long-Term Functional Outcome and Mortality than High-Sensitivity C-Reactive Protein in Patients with Ischemic Stroke. Mol Neurobiol 53, 1509–1517 (2016). https://doi.org/10.1007/s12035-015-9112-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9112-7