Abstract
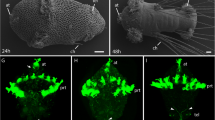
Acetylcholinesterase (ACHE) is a glycoprotein with a key role in terminating synaptic transmission in cholinergic neurons of both vertebrates and invertebrates. ACHE is also involved in the regulation of cell growth and morphogenesis during embryogenesis and regeneration acting through its non-cholinergic sites. The mollusk Octopus vulgaris provides a powerful model for investigating the mechanisms underlying tissue morphogenesis due to its high regenerative power. Here, we performed a comparative investigation of arm morphogenesis during adult arm regeneration and embryonic arm development which may provide insights on the conserved ACHE pathways. In this study, we cloned and characterized O. vulgaris ACHE, finding a single highly conserved ACHE hydrophobic variant, characterized by prototypical catalytic sites and a putative consensus region for a glycosylphosphatidylinositol (GPI)-anchor attachment at the COOH-terminus. We then show that its expression level is correlated to the stage of morphogenesis in both adult and embryonic arm. In particular, ACHE is localized in typical neuronal sites when adult-like arm morphology is established and in differentiating cell locations during the early stages of arm morphogenesis. This possibility is also supported by the presence in the ACHE sequence and model structure of both cholinergic and non-cholinergic sites. This study provides insights into ACHE conserved roles during processes of arm morphogenesis. In addition, our modeling study offers a solid basis for predicting the interaction of the ACHE domains with pharmacological blockers for in vivo investigations. We therefore suggest ACHE as a target for the regulation of tissue morphogenesis.







Similar content being viewed by others
Abbreviations
- ACHE:
-
Acetylcholinesterase
- ASW:
-
Artificial sea water
- BCHE:
-
Butyrylcholinesterase
- CAS:
-
Catalytic anionic site
- CAT:
-
Catalytic triad
- GPI:
-
Glycosylphosphatidylinositol
- ORF:
-
Open reading frame
- PAS:
-
Peripheral binding site
- RMSD:
-
Root mean square deviation
- RT-qPCR:
-
Real-time quantitative PCR
References
Jiang H, Zhang XJ (2008) Acetylcholinesterase and apoptosis. A novel perspective for an old enzyme. FEBS J 275(4):612–617. doi:10.1111/j.1742-4658.2007.06236.x
Soreq H, Seidman S (2001) Acetylcholinesterase—new roles for an old actor. Nat Rev Neurosci 2:294–302
Layer PG, Willbold E (1995) Novel functions of cholinesterases in development, physiology and disease. Prog Histochem Cytochem 29(3):1–94
Singer M, Davis MH, Arkowitz ES (1960) Acetylcholinesterase activity in the regenerating forelimb of the adult newt, triturus. J Embryol Exp Morpholog 8:98–111
Lenique PM, Feral JP (1976) A mechanism of action of neurotransmitters on the regeneration of the planarian worm Dugesia tigrina. Role of acetylcholine as a negative feed-back. Acta Zool 57:1–5
Srivatsan M, Peretz B (1997) Acetylcholinesterase promotes regeneration of neurites in cultured adult neurons of Aplysia. Neuroscience 77(3):921–931
Lauder JM, Schambra UB (1999) Morphogenetic roles of acetylcholine. Environ Health Perspect 107:65–69
Vogel-Hopker A, Sperling LE, Layer PG (2012) Co-opting functions of cholinesterases in neural, limb and stem cell development. Protein Pept Lett 19(2):155–164
Dori A, Soreq H (2006) ARP, the cleavable C-terminal peptide of “readthrough” acetylcholinesterase, promotes neuronal development and plasticity. J Mol Neurosci MN 28(3):247–255. doi:10.1385/JMN:28:3:247
Paraoanu LE, Steinert G, Klaczinski J, Becker-Rock M, Bytyqi A, Layer PG (2006) On functions of cholinesterases during embryonic development. J Mol Neurosci MN 30(1–2):201–204. doi:10.1385/JMN:30:1:201
Falugi C, Aluigi MG (2012) Early appearance and possible functions of non-neuromuscular cholinesterase activities. Front Mol Neurosci 5:54. doi:10.3389/fnmol.2012.00054
Behra M, Cousin X, Bertrand C, Vonesch JL, Biellmann D, Chatonnet A, Strähle U (2002) Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo. Nat Neurosci 5:111–118
Berg L, Andersson CD, Artursson E, Hornberg A, Tunemalm AK, Linusson A, Ekstrom F (2011) Targeting acetylcholinesterase: identification of chemical leads by high throughput screening, structure determination and molecular modeling. PLoS One 6(11):e26039. doi:10.1371/journal.pone.0026039
Massoulié J, Anselmet A, Bon S, Krejci E, Legay C, Morel N, Simon S (1999) The polymorphism of acetylcholinesterase: post-translational processing, quaternary associations and localization. Chem Biol Interact 119–120:29–42
Massoulié J (2002) The origin of the molecular diversity and functional anchoring of cholinesterases. Neuro Signals 11(3):130–143
Hicks D, John D, Makova NZ, Henderson Z, Nalivaeva NN, Turner AJ (2011) Membrane targeting, shedding and protein interactions of brain acetylcholinesterase. J Neurochem 116(5):742–746. doi:10.1111/j.1471-4159.2010.07032.x
Dvir H, Jiang HL, Wong DM, Harel M, Chetrit M, He XC, Jin GY, Yu GL, Tang XC, Silman I, Bai DL, Sussman JL (2002) X-ray structures of Torpedo californica acetylcholinesterase complexed with (+)-huperzine A and (−)-huperzine B: structural evidence for an active site rearrangement. Biochemistry 41(35):10810–10818
Pezzementi L, Johnson K, Tsigelny I, Cotney J, Manning E, Barker A, Merritt S (2003) Amino acids defining the acyl pocket of an invertebrate cholinesterase. Comp Biochem Physiol B Biochem Mol Biol 136(4):813–832
Silman I, Sussman JL (2008) Acetylcholinesterase: how is structure related to function? Chem Biol Interact 175(1–3):3–10. doi:10.1016/j.cbi.2008.05.035
Pierleoni A, Martelli PL, Casadio R (2008) PredGPI: a GPI-anchor predictor. BMC Bioinforma 9:392. doi:10.1186/1471-2105-9-392
Paz A, Xie Q, Greenblatt HM, Fu W, Tang Y, Silman I, Qiu Z, Sussman JL (2009) The crystal structure of a complex of acetylcholinesterase with a bis-(-)-nor-meptazinol derivative reveals disruption of the catalytic triad. J Med Chem 52(8):2543–2549. doi:10.1021/jm801657v
Dvir H, Silman I, Harel M, Rosenberry TL, Sussman JL (2010) Acetylcholinesterase: from 3D structure to function. Chem Biol Interact 187(1–3):10–22. doi:10.1016/j.cbi.2010.01.042
Pegan K, Matkovic U, Mars T, Mis K, Pirkmajer S, Brecelj J, Grubic Z (2010) Acetylcholinesterase is involved in apoptosis in the precursors of human muscle regeneration. Chem Biol Interact 187:96–100
Xie J, Jiang H, Wan YH, Du AY, Guo KJ, Liu T, Ye WY, Niu X, Wu J, Dong XQ, Zhang XJ (2011) Induction of a 55 kDa acetylcholinesterase protein during apoptosis and its negative regulation by the Akt pathway. J Mol Cell Biol 3(4):250–259. doi:10.1093/jmcb/mjq047
Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I (1991) Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science 253(5022):872–879
Shafferman A, Kronman C, Flashner Y, Leitner M, Grosfeld H, Ordentlich A, Gozes Y, Cohen S, Ariel N, Barak D et al (1992) Mutagenesis of human acetylcholinesterase. Identification of residues involved in catalytic activity and in polypeptide folding. J Biol Chem 267(25):17640–17648
Fiorito G, Affuso A, Anderson DB, Basil J, Bonnaud L, Botta G, Cole A, D’Angelo L, De Girolamo P, Dennison N, Dickel L, Di Cosmo A, Di Cristo C, Gestal C, Fonseca R, Grasso F, Kristiansen T, Kuba M, Maffucci F, Manciocco A, Mark FC, Melillo D, Osorio D, Palumbo A, Perkins K, Ponte G, Raspa M, Shashar N, Smith J, Smith D, Sykes A, Villanueva R, Tublitz N, Zullo L, Andrews P (2014) Cephalopods in neuroscience: regulations, research and the 3Rs. Invert Neurosci IN. doi:10.1007/s10158-013-0165-x
Arnold JM (1965) Normal embryonic stages of the squid, Loligo pealii (Lesueur). Biol Bull 128(1):24–32
Xu Y, Colletier JP, Weik M, Jiang H, Moult J, Silman I, Sussman JL (2008) Flexibility of aromatic residues in the active-site gorge of acetylcholinesterase: X-ray versus molecular dynamics. Biophys J 95(5):2500–2511. doi:10.1529/biophysj.108.129601
Eswar N, John B, Mirkovic N, Fiser A, Ilyin VA, Pieper U, Stuart AC, Marti-Renom MA, Madhusudhan MS, Yerkovich B, Sali A (2003) Tools for comparative protein structure modeling and analysis. Nucleic Acids Res 31(13):3375–3380
Shen MY, Sali A (2006) Statistical potential for assessment and prediction of protein structures. Protein Sci Publ Protein Soc 15(11):2507–2524. doi:10.1110/ps.062416606
Mackerell ADJ, Feig M, Brooks CL (2004) 3rd Extending the treatment of backbone energetics in protein force fields: limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J Comput Chem 25:1400–1415
Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K (2005) Scalable molecular dynamics with NAMD. J Comput Chem 26(16):1781–1802. doi:10.1002/jcc.20289
Sirakov M, Zarrella I, Borra M, Rizzo F, Biffali E, Arnone MI, Fiorito G (2009) Selection and validation of a set of reliable reference genes for quantitative RT-PCR studies in the brain of the Cephalopod Mollusc Octopus vulgaris. BMC Mol Biol 10:70. doi:10.1186/1471-2199-10-70
Tiveron MC, Hirsch MR, Brunet JF (1996) The expression pattern of the transcription factor Phox2 delineates synaptic pathways of the autonomic nervous system. J Neurosci Off J Soc Neurosci 16(23):7649–7660
Lee PN, Callaerts P, De Couet HG, Martindale MQ (2003) Cephalopod Hox genes and the origin of morphological novelties. Nature 424(6952):1061–1065. doi:10.1038/nature01872
Talesa V, Grauso M, Arpagaus M, Giovannini E, Romani R, Rosi G (1999) Molecular cloning and expression of a full-length cDNA encoding acetylcholinesterase in optic lobes of the squid Loligo opalescens: a new member of the cholinesterase family resistant to diisopropyl fluorophosphate. J Neurochem 72(3):1250–1258
Harel M, Kryger G, Rosenberry TL, Mallender WD, Lewis T, Fletcher RJ, Guss JM, Silman I, Sussman JL (2000) Three-dimensional structures of Drosophila melanogaster acetylcholinesterase and of its complexes with two potent inhibitors. Protein Sci Publ Protein Soc 9(6):1063–1072. doi:10.1110/ps.9.6.1063
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425
Guindon S, Lethiec F, Duroux P, Gascuel O (2005) PHYML online—a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Res 33(Web Server issue):W557–W559. doi:10.1093/nar/gki352
Pang YP (2006) Novel acetylcholinesterase target site for malaria mosquito control. PLoS One 1:e58. doi:10.1371/journal.pone.0000058
Fossati SM, Carella F, De Vico G, Benfenati F, Zullo L (2013) Octopus arm regeneration: role of acetylcholinesterase during morphological modification. JEMBE 447:93–99
Futerman AH, Low MG, Ackermann KE, Sherman WR, Silman I (1985) Identification of covalently bound inositol in the hydrophobic membrane-anchoring domain of Torpedo acetylcholinesterase. Biochem Biophys Res Commun 129(1):312–317
Massoulié J, Perrier N, Noureddine H, Liang D, Bon S (2008) Old and new questions about cholinesterases. Chem Biol Interact 175:30–44
Taylor P (1991) The cholinesterases. J Biol Chem 266(7):4025–4028
Massoulié J, Pezzementi L, Bon S, Krejci E, Vallette FM (1993) Molecular and cellular biology of cholinesterases. Prog Neurobiol 41(1):31–91
Harel M, Kleywegt GJ, Ravelli RB, Silman I, Sussman JL (1995) Crystal structure of an acetylcholinesterase-fasciculin complex: interaction of a three-fingered toxin from snake venom with its target. Structure 3(12):1355–1366
Grisaru D, Sternfeld M, Eldor A, Glick D, Soreq H (1999) Structural roles of acetylcholinesterase variants in biology and pathology. Eur J Biochem/FEBS 264(3):672–686
Massoulie J, Anselmet A, Bon S, Krejci E, Legay C, Morel N, Simon S (1998) Acetylcholinesterase: C-terminal domains, molecular forms and functional localization. J Physiol Paris 92(3–4):183–190
Grauso M, Culetto E, Combes D, Fedon Y, Toutant JP, Arpagaus M (1998) Existence of four acetylcholinesterase genes in the nematodes Caenorhabditis elegans and Caenorhabditis briggsae. FEBS Lett 424(3):279–284
Combes D, Fedon Y, Grauso M, Toutant JP, Arpagaus M (2000) Four genes encode acetylcholinesterases in the nematodes Caenorhabditis elegans and Caenorhabditis briggsae. cDNA sequences, genomic structures, mutations and in vivo expression. J Mol Biol 300(4):727–742. doi:10.1006/jmbi.2000.3917
Cossu G, Eusebi F, Grassi F, Wanke E (1987) Acetylcholine receptor channels are present in undifferentiated satellite cells but not in embryonic myoblasts in culture. Dev Biol 123(1):43–50
Yamamoto M, Shimazaki Y, Shigeno S (2003) Atlas of the embryonic brain in the pygmy squid, Idiosepius paradoxus. Zool Sci 20(2):163–179
Acknowledgments
We thank Jennifer Helm for the help with data collection and Andrea Contestabile for the technical support. We are also grateful to Prof. Jenny Kien for the suggestions and editorial assistance.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fossati, S.M., Candiani, S., Nödl, MT. et al. Identification and Expression of Acetylcholinesterase in Octopus vulgaris Arm Development and Regeneration: a Conserved Role for ACHE?. Mol Neurobiol 52, 45–56 (2015). https://doi.org/10.1007/s12035-014-8842-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8842-2