Abstract
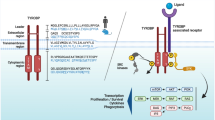
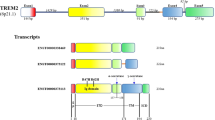
Recently, studies have provided convincing data that TYRO protein tyrosine kinase-binding protein (TYROBP), a key regulator in immune systems, is significantly upregulated in the brain of patients with Alzheimer’s disease (AD). TYROBP acts as a signaling adaptor protein for numerous cell surface receptors, playing important roles in signal transduction in dendritic cells, osteoclasts, macrophages, and microglia. Although several TYROBP-related cell surface receptors including triggering receptor expressed on myeloid 2 (TREM2), signal regulatory protein β1 (SIRPβ1), and complement receptor 3 (CR3) were found to participate in the pathogenesis of AD, the role of TYROBP in AD still remains elusive. Emerging piece of evidence has demonstrated that TYROBP could enhance phagocytic activity of microglia, which is responsible for the clearance of amyloid-β (Aβ) peptides and apoptotic neurons. TYROBP also participates in suppression of inflammatory responses by repression of microglia-mediated cytokine production and secretion. In this article, we introduce the structure, localization, and function of TYROBP. Meanwhile, we review recent articles concerning the association of TYROBP and its related receptors with AD pathogenesis and speculate the possible roles of TYROBP in this disease. Based on the potential protective actions of TYROBP in AD pathogenesis, targeting TYROBP might provide new opportunities for AD treatment.



Similar content being viewed by others
References
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science (New York, NY) 297(5580):353–356. doi:10.1126/science.1072994
Jiang T, Yu JT, Tian Y, Tan L (2013) Epidemiology and etiology of Alzheimer’s disease: from genetic to non-genetic factors. Current Alzheimer Research 10(8):852–867
Perry VH, Nicoll JA, Holmes C (2010) Microglia in neurodegenerative disease. Nat Rev Neurol 6(4):193–201. doi:10.1038/nrneurol.2010.17
Streit WJ, Mrak RE, Griffin WS (2004) Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation 1(1):14. doi:10.1186/1742-2094-1-14
Jiang T, Yu JT, Tan L (2012) Novel disease-modifying therapies for Alzheimer’s disease. Journal of Alzheimer’s Disease : JAD 31(3):475–492. doi:10.3233/jad-2012-120640
Yu JT, Tan L, Hardy J (2014) Apolipoprotein E in Alzheimer’s disease: an update. Annual review of neuroscience. doi:10.1146/annurev-neuro-071013-014300
Zhang B, Gaiteri C, Bodea LG, Wang Z, McElwee J, Podtelezhnikov AA, Zhang C, Xie T, Tran L, Dobrin R, Fluder E, Clurman B, Melquist S, Narayanan M, Suver C, Shah H, Mahajan M, Gillis T, Mysore J, MacDonald ME, Lamb JR, Bennett DA, Molony C, Stone DJ, Gudnason V, Myers AJ, Schadt EE, Neumann H, Zhu J, Emilsson V (2013) Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 153(3):707–720. doi:10.1016/j.cell.2013.03.030
Tomasello E, Vivier E (2005) KARAP/DAP12/TYROBP: three names and a multiplicity of biological functions. Eur J Immunol 35(6):1670–1677. doi:10.1002/eji.200425932
Kiialainen A, Hovanes K, Paloneva J, Kopra O, Peltonen L (2005) Dap12 and Trem2, molecules involved in innate immunity and neurodegeneration, are co-expressed in the CNS. Neurobiol Dis 18(2):314–322. doi:10.1016/j.nbd.2004.09.007
Bouchon A, Hernandez-Munain C, Cella M, Colonna M (2001) A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. The Journal of experimental medicine 194(8):1111–1122
Takaki R, Watson SR, Lanier LL (2006) DAP12: an adapter protein with dual functionality. Immunol Rev 214:118–129. doi:10.1111/j.1600-065X.2006.00466.x
Turnbull IR, Colonna M (2007) Activating and inhibitory functions of DAP12. Nat Rev Immunol 7(2):155–161. doi:10.1038/nri2014
Peng Q, Long CL, Malhotra S, Humphrey MB (2013) A physical interaction between the adaptor proteins DOK3 and DAP12 is required to inhibit lipopolysaccharide signaling in macrophages. Sci Signal 6(289):72. doi:10.1126/scisignal.2003801
Colonna M (2003) TREMs in the immune system and beyond. Nat Rev Immunol 3(6):445–453. doi:10.1038/nri1106
Hamerman JA, Ni M, Killebrew JR, Chu CL, Lowell CA (2009) The expanding roles of ITAM adapters FcRgamma and DAP12 in myeloid cells. Immunol Rev 232(1):42–58. doi:10.1111/j.1600-065X.2009.00841.x
Tomasello E, Olcese L, Vely F, Geourgeon C, Blery M, Moqrich A, Gautheret D, Djabali M, Mattei MG, Vivier E (1998) Gene structure, expression pattern, and biological activity of mouse killer cell activating receptor-associated protein (KARAP)/DAP-12. The Journal of biological chemistry 273(51):34115–34119
Paloneva J, Kestila M, Wu J, Salminen A, Bohling T, Ruotsalainen V, Hakola P, Bakker AB, Phillips JH, Pekkarinen P, Lanier LL, Timonen T, Peltonen L (2000) Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet 25(3):357–361. doi:10.1038/77153
Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH (1998) Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature 391(6668):703–707. doi:10.1038/35642
Lanier LL, Bakker AB (2000) The ITAM-bearing transmembrane adaptor DAP12 in lymphoid and myeloid cell function. Immunol Today 21(12):611–614
Lanier LL (2009) DAP10- and DAP12-associated receptors in innate immunity. Immunol Rev 227(1):150–160. doi:10.1111/j.1600-065X.2008.00720.x
Colonna M (2003) DAP12 signaling: from immune cells to bone modeling and brain myelination. J Clin Invest 111(3):313–314. doi:10.1172/jci17745
Mocsai A, Abram CL, Jakus Z, Hu Y, Lanier LL, Lowell CA (2006) Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat Immunol 7(12):1326–1333. doi:10.1038/ni1407
Cao H, Lakner U, de BB, Traherne JA, Trowsdale J, Barrow AD (2008) SIGLEC16 encodes a DAP12-associated receptor expressed in macrophages that evolved from its inhibitory counterpart SIGLEC11 and has functional and non-functional alleles in humans. Eur J Immunol JT - European journal of immunology 38 (8):2303-2315 LID - 2310.1002/eji.200738078 [doi]. doi:10.1002/eji.200738078
Varnum MM, Ikezu T (2012) The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer’s disease brain. Arch Immunol Ther Exp 60(4):251–266. doi:10.1007/s00005-012-0181-2
Hanisch UK, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10(11):1387–1394. doi:10.1038/nn1997
Sessa G, Podini P, Mariani M, Meroni A, Spreafico R, Sinigaglia F, Colonna M, Panina P, Meldolesi J (2004) Distribution and signaling of TREM2/DAP12, the receptor system mutated in human polycystic lipomembraneous osteodysplasia with sclerosing leukoencephalopathy dementia. The European journal of neuroscience 20(10):2617–2628. doi:10.1111/j.1460-9568.2004.03729.x
Satoh J, Shimamura Y, Tabunoki H (2012) Gene expression profile of THP-1 monocytes following knockdown of DAP12, a causative gene for Nasu-Hakola disease. Cell Mol Neurobiol 32(3):337–343. doi:10.1007/s10571-011-9769-z
Hickman SE, El Khoury J (2013) TREM2 and the neuroimmunology of Alzheimer’s disease. Biochemical pharmacology. doi:10.1016/j.bcp.2013.11.021
Hsieh CL, Koike M, Spusta SC, Niemi EC, Yenari M, Nakamura MC, Seaman WE (2009) A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J Neurochem 109(4):1144–1156. doi:10.1111/j.1471-4159.2009.06042.x
Takahashi K, Rochford CD, Neumann H (2005) Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. The Journal of experimental medicine 201(4):647–657. doi:10.1084/jem.20041611
Ito H, Hamerman JA (2012) TREM-2, triggering receptor expressed on myeloid cell-2, negatively regulates TLR responses in dendritic cells. Eur J Immunol 42(1):176–185. doi:10.1002/eji.201141679
Bajramovic JJ (2011) Regulation of innate immune responses in the central nervous system. CNS & neurological disorders drug targets 10(1):4–24
Guerreiro R, Hardy J (2013) TREM2 and neurodegenerative disease. N Engl J Med 369(16):1569–1570
Neumann H, Daly MJ (2013) Variant TREM2 as risk factor for Alzheimer’s disease. N Engl J Med 368(2):182–184. doi:10.1056/NEJMe1213157
Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H (2007) TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med 4(4):e124. doi:10.1371/journal.pmed.0040124
Neumann H, Takahashi K (2007) Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. J Neuroimmunol 184(1–2):92–99. doi:10.1016/j.jneuroim.2006.11.032
Yu JT, Jiang T, Wang YL, Wang HF, Zhang W, Hu N, Tan L, Sun L, Tan MS, Zhu XC, Tan L (2014) Triggering receptor expressed on myeloid cells 2 variant is rare in late-onset Alzheimer’s disease in Han Chinese individuals. Neurobiology of aging 35 (4):937 e931-933. doi:10.1016/j.neurobiolaging.2013.10.075
Melchior B, Garcia AE, Hsiung BK, Lo KM, Doose JM, Thrash JC, Stalder AK, Staufenbiel M, Neumann H, Carson MJ (2010) Dual induction of TREM2 and tolerance-related transcript, Tmem176b, in amyloid transgenic mice: implications for vaccine-based therapies for Alzheimer’s disease. ASN neuro 2(3):e00037. doi:10.1042/AN20100010
Jiang T, Yu JT, Zhu XC, Tan MS, Gu LZ, Zhang YD, Tan L (2013) Triggering receptor expressed on myeloid cells 2 knockdown exacerbates aging-related neuroinflammation and cognitive deficiency in senescence-accelerated mouse prone 8 mice. Neurobiology of aging. doi:10.1016/j.neurobiolaging.2013.11.026
Hu N, Tan MS, Yu JT, Sun L, Tan L, Wang YL, Jiang T, Tan L (2014) Increased expression of TREM2 in peripheral blood of Alzheimer’s disease patients. Journal of Alzheimer’s Disease : JAD 38(3):497–501. doi:10.3233/jad-130854
Gaikwad S, Larionov S, Wang Y, Dannenberg H, Matozaki T, Monsonego A, Thal DR, Neumann H (2009) Signal regulatory protein-beta1: a microglial modulator of phagocytosis in Alzheimer’s disease. The American journal of pathology 175(6):2528–2539. doi:10.2353/ajpath.2009.090147
Maier M, Peng Y, Jiang L, Seabrook TJ, Carroll MC, Lemere CA (2008) Complement C3 deficiency leads to accelerated amyloid beta plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 28(25):6333–6341. doi:10.1523/jneurosci.0829-08.2008
Lucin KM, Wyss-Coray T (2009) Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 64(1):110–122. doi:10.1016/j.neuron.2009.08.039
McGeer PL, Akiyama H, Itagaki S, McGeer EG (1989) Activation of the classical complement pathway in brain tissue of Alzheimer patients. Neurosci Lett 107(1–3):341–346
Webster S, Lue LF, Brachova L, Tenner AJ, McGeer PL, Terai K, Walker DG, Bradt B, Cooper NR, Rogers J (1997) Molecular and cellular characterization of the membrane attack complex, C5b-9, in Alzheimer’s disease. Neurobiol Aging 18(4):415–421
Frank S, Burbach GJ, Bonin M, Walter M, Streit W, Bechmann I, Deller T (2008) TREM2 is upregulated in amyloid plaque-associated microglia in aged APP23 transgenic mice. Glia 56(13):1438–1447. doi:10.1002/glia.20710
Coskun PE, Beal MF, Wallace DC (2004) Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci U S A 101(29):10726–10731. doi:10.1073/pnas.0403649101
Webber KM, Raina AK, Marlatt MW, Zhu X, Prat MI, Morelli L, Casadesus G, Perry G, Smith MA (2005) The cell cycle in Alzheimer disease: a unique target for neuropharmacology. Mech Ageing Dev 126(10):1019–1025. doi:10.1016/j.mad.2005.03.024
Paloneva J, Autti T, Hakola P, Haltia MJ (1993) Polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLOSL). In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K (eds) GeneReviews. University of Washington, Seattle
Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R, Salmaggi A, Tranebjaerg L, Konttinen Y, Peltonen L (2002) Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet 71(3):656–662. doi:10.1086/342259
Thrash JC, Torbett BE, Carson MJ (2009) Developmental regulation of TREM2 and DAP12 expression in the murine CNS: implications for Nasu-Hakola disease. Neurochem Res 34(1):38–45. doi:10.1007/s11064-008-9657-1
Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT (2013) NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493(7434):674–678. doi:10.1038/nature11729
Nataf S, Anginot A, Vuaillat C, Malaval L, Fodil N, Chereul E, Langlois JB, Dumontel C, Cavillon G, Confavreux C, Mazzorana M, Vico L, Belin MF, Vivier E, Tomasello E, Jurdic P (2005) Brain and bone damage in KARAP/DAP12 loss-of-function mice correlate with alterations in microglia and osteoclast lineages. The American journal of pathology 166(1):275–286. doi:10.1016/s0002-9440(10)62251-1
Hamerman JA, Tchao NK, Lowell CA, Lanier LL (2005) Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol 6(6):579–586. doi:10.1038/ni1204
Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL (2006) Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. Journal of immunology (Baltimore, Md : 1950) 177(4):2051–2055
Turnbull IR, Gilfillan S, Cella M, Aoshi T, Miller M, Piccio L, Hernandez M, Colonna M (2006) Cutting edge: TREM-2 attenuates macrophage activation. Journal of immunology (Baltimore, Md : 1950) 177(6):3520–3524
Long C, Peng QS, Malhotra S, Humphrey M (2013) DAP12 inhibition of LPS signaling in macrophages is mediated by DOK3. J Immunol 190
Schadt EE (2009) Molecular networks as sensors and drivers of common human diseases. Nature 461(7261):218–223. doi:10.1038/nature08454
Acknowledgements
This work was supported by the grants from the National Natural Science Foundation of China to L.T. (81171209, 81371406) and J.T.Y. (81000544), the grants from the Shandong Provincial Natural Science Foundation to L.T. (ZR2011HZ001) and J.T.Y. (ZR2010HQ004), the Medicine and Health Science Technology Development Project of Shandong Province to L.T. (2011WSA02018) and J.T.Y. (2011WSA02020), and the Innovation Project for Postgraduates of Jiangsu Province to T.J. (CXLX13_561).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jing Ma and Teng Jiang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ma, J., Jiang, T., Tan, L. et al. TYROBP in Alzheimer’s Disease. Mol Neurobiol 51, 820–826 (2015). https://doi.org/10.1007/s12035-014-8811-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8811-9