Abstract
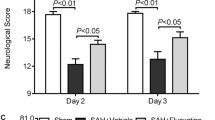
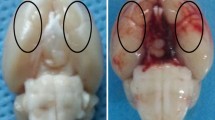
Subarachnoid hemorrhage (SAH) causes brain injury via glutamate excitotoxicity, which leads to an excessive Ca2+ influx and this starts an apoptotic cascade. Memantine has been proven to reduce brain injury in several types of brain insults. This study investigated the neuro-protective potential of memantine after SAH and explored the underlying mechanisms. An endovascular perforation rat model of SAH was used and Sprague–Dawley rats were randomized into sham surgery, SAH + vehicle, and SAH + memantine groups. The effects of memantine on SAH were evaluated by assessing the neuro-behavioral functions, blood–brain barrier (BBB) permeability and neuronal cell preservation. The mechanisms of action of memantine, with its N-methyl-d-aspartate (NMDA) antagonistic characteristics on nitric oxide synthase (NOS) expression and peroxynitrite formation, were also investigated. The apoptotic cascade after SAH was suppressed by memantine. Neuronal NOS (nNOS) expression, peroxynitrite formation, and subsequent oxidative/nitrosative stress were also reduced. Memantine effectively preserved BBB integrity, rescued neuronal injury, and improved neurological outcome in experimental SAH. Memantine has neuro-protective potential in experimental SAH and may help combat SAH-induced brain damage in the future.







Similar content being viewed by others
References
Macdonald RL, Cusimano MD, Etminan N, Hanggi D, Hasan D, Ilodigwe D, Jaja B, Lantigua H, Le Roux P, Lo B, Louffat-Olivares A, Mayer S, Molyneux A, Quinn A, Schweizer TA, Schenk T, Spears J, Todd M, Torner J, Vergouwen MD, Wong GK, Collaboration S (2013) Subarachnoid Hemorrhage International Trialists data repository (SAHIT). World Neurosurg 79(3–4):418–422
King JT Jr (1997) Epidemiology of aneurysmal subarachnoid hemorrhage. Neuroimaging Clin N Am 7(4):659–668
Cahill J, Calvert JW, Zhang JH (2006) Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 26(11):1341–1353
Sarrafzadeh A, Haux D, Kuchler I, Lanksch WR, Unterberg AW (2004) Poor-grade aneurysmal subarachnoid hemorrhage: relationship of cerebral metabolism to outcome. J Neurosurg 100(3):400–406
Hillered L, Vespa PM, Hovda DA (2005) Translational neurochemical research in acute human brain injury: the current status and potential future for cerebral microdialysis. J Neurotrauma 22(1):3–41
Arundine M, Tymianski M (2003) Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 34(4–5):325–337
Schulz MK, Wang LP, Tange M, Bjerre P (2000) Cerebral microdialysis monitoring: determination of normal and ischemic cerebral metabolisms in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg 93(5):808–814
Park S, Yamaguchi M, Zhou C, Calvert JW, Tang J, Zhang JH (2004) Neurovascular protection reduces early brain injury after subarachnoid hemorrhage. Stroke 35(10):2412–2417
Sehba FA, Chereshnev I, Maayani S, Friedrich V Jr, Bederson JB (2004) Nitric oxide synthase in acute alteration of nitric oxide levels after subarachnoid hemorrhage. Neurosurgery 55(3):671–677, discussion 677–678
Dawson VL, Kizushi VM, Huang PL, Snyder SH, Dawson TM (1996) Resistance to neurotoxicity in cortical cultures from neuronal nitric oxide synthase-deficient mice. J Neurosci 16(8):2479–2487
Rammes G, Danysz W, Parsons CG (2008) Pharmacodynamics of memantine: an update. Curr Neuropharmacol 6(1):55–78
Chen HS, Wang YF, Rayudu PV, Edgecomb P, Neill JC, Segal MM, Lipton SA, Jensen FE (1998) Neuroprotective concentrations of the N-methyl-d-aspartate open-channel blocker memantine are effective without cytoplasmic vacuolation following post-ischemic administration and do not block maze learning or long-term potentiation. Neuroscience 86(4):1121–1132
Parsons CG, Danysz W, Quack G (1999) Memantine is a clinically well tolerated N-methyl-d-aspartate (NMDA) receptor antagonist—a review of preclinical data. Neuropharmacology 38(6):735–767
Ehrlich M, Knolle E, Ciovica R, Bock P, Turkof E, Grabenwoger M, Cartes-Zumelzu F, Kocher A, Pockberger H, Fang WC, Wolner E, Havel M (1999) Memantine for prevention of spinal cord injury in a rabbit model. J Thorac Cardiovasc Surg 117(2):285–291
Macleod MR, Fisher M, O'Collins V, Sena ES, Dirnagl U, Bath PM, Buchan A, van der Worp HB, Traystman R, Minematsu K, Donnan GA, Howells DW (2009) Good laboratory practice: preventing introduction of bias at the bench. Stroke 40(3):e50–e52
Bederson JB, Germano IM, Guarino L (1995) Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke 26(6):1086–1091, discussion 1091–1082
Thal SC, Sporer S, Klopotowski M, Thal SE, Woitzik J, Schmid-Elsaesser R, Plesnila N, Zausinger S (2009) Brain edema formation and neurological impairment after subarachnoid hemorrhage in rats. Laboratory investigation. J Neurosurg 111(5):988–994
Sugawara T, Ayer R, Jadhav V, Zhang JH (2008) A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods 167(2):327–334
Takata K, Sheng H, Borel CO, Laskowitz DT, Warner DS, Lombard FW (2009) Simvastatin treatment duration and cognitive preservation in experimental subarachnoid hemorrhage. J Neurosurg Anesthesiol 21(4):326–333
Silasi G, Colbourne F (2009) Long-term assessment of motor and cognitive behaviours in the intraluminal perforation model of subarachnoid hemorrhage in rats. Behav Brain Res 198(2):380–387
Germano A, Caffo M, Angileri FF, Arcadi F, Newcomb-Fernandez J, Caruso G, Meli F, Pineda JA, Lewis SB, Wang KK, Bramanti P, Costa C, Hayes RL (2007) NMDA receptor antagonist felbamate reduces behavioral deficits and blood–brain barrier permeability changes after experimental subarachnoid hemorrhage in the rat. J Neurotrauma 24(4):732–744
Lin KY, Cherng CG, Yang FR, Lin LC, Lu RB, Yu L (2011) Memantine abolishes the formation of cocaine-induced conditioned place preference possibly via its IL-6-modulating effect in medial prefrontal cortex. Behav Brain Res 220(1):126–131
Quintard H, Borsotto M, Veyssiere J, Gandin C, Labbal F, Widmann C, Lazdunski M, Heurteaux C (2011) MLC901, a traditional Chinese medicine protects the brain against global ischemia. Neuropharmacology 61(4):622–631
Belayev L, Busto R, Zhao W, Ginsberg MD (1996) Quantitative evaluation of blood–brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res 739(1–2):88–96
Bredt DS, Snyder SH (1989) Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A 86(22):9030–9033
Khan J, Brennand DM, Bradley N, Gao B, Bruckdorfer R, Jacobs M (1998) 3-Nitrotyrosine in the proteins of human plasma determined by an ELISA method. Biochem J 330(Pt 2):795–801
Juan WS, Lin HW, Chen YH, Chen HY, Hung YC, Tai SH, Huang SY, Chen TY, Lee EJ (2012) Optimal Percoll concentration facilitates flow cytometric analysis for annexin V/propidium iodine-stained ischemic brain tissues. Cytometry Part A 81(5):400–408
Westermaier T, Jauss A, Eriskat J, Kunze E, Roosen K (2009) Time-course of cerebral perfusion and tissue oxygenation in the first 6 h after experimental subarachnoid hemorrhage in rats. J Cereb Blood Flow Metab 29(4):771–779
Germano A, d'Avella D, Imperatore C, Caruso G, Tomasello F (2000) Time-course of blood–brain barrier permeability changes after experimental subarachnoid haemorrhage. Acta Neurochir 142(5):575–580, discussion 580–571
Jeon H, Ai J, Sabri M, Tariq A, Shang X, Chen G, Macdonald RL (2009) Neurological and neurobehavioral assessment of experimental subarachnoid hemorrhage. BMC Neurosci 10:103
Lipton SA (2004) Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx 1(1):101–110
Zuccarello M, Lewis AI, Upputuri S, Farmer JB, Anderson DK (1994) Effect of remacemide hydrochloride on subarachnoid hemorrhage-induced vasospasm in rabbits. J Neurotrauma 11(6):691–698
Dyker AG, Lees KR (1999) Remacemide hydrochloride: a double-blind, placebo-controlled, safety and tolerability study in patients with acute ischemic stroke. Stroke 30(9):1796–1801
Tribut O, Bentue-Ferrer D, Verdier MC, le groupe Suivi Therapeutique Pharmacologique de la Societe Francaise de Pharmacologie et de T (2010) Therapeutic drug monitoring of felbamate. Therapie 65(1):35–38
Xia P, Chen HS, Zhang D, Lipton SA (2010) Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J Neurosci 30(33):11246–11250
Block F, Schwarz M (1996) Memantine reduces functional and morphological consequences induced by global ischemia in rats. Neurosci Lett 208(1):41–44
Frankiewicz T, Parsons CG (1999) Memantine restores long term potentiation impaired by tonic N-methyl-d-aspartate (NMDA) receptor activation following reduction of Mg2+ in hippocampal slices. Neuropharmacology 38(9):1253–1259
Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ, Memantine Study G (2003) Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med 348(14):1333–1341
Wilcock G, Mobius HJ, Stoffler A, group MMM (2002) A double-blind, placebo-controlled multicentre study of memantine in mild to moderate vascular dementia (MMM500). Int Clin Psychopharmacol 17(6):297–305
Sabri M, Kawashima A, Ai J, Macdonald RL (2008) Neuronal and astrocytic apoptosis after subarachnoid hemorrhage: a possible cause for poor prognosis. Brain Res 1238:163–171
Seif el Nasr M, Peruche B, Rossberg C, Mennel HD, Krieglstein J (1990) Neuroprotective effect of memantine demonstrated in vivo and in vitro. Eur J Pharmacol 185(1):19–24
Duan Y, Gross RA, Sheu SS (2007) Ca2+-dependent generation of mitochondrial reactive oxygen species serves as a signal for poly(ADP-ribose) polymerase-1 activation during glutamate excitotoxicity. J Physiol 585(Pt 3):741–758
Sharp CD, Houghton J, Elrod JW, Warren A, Jackson TH, Jawahar A, Nanda A, Minagar A, Alexander JS (2005) N-methyl-d-aspartate receptor activation in human cerebral endothelium promotes intracellular oxidant stress. Am J Physiol Heart Circ Physiol 288(4):H1893–H1899
Scott GS, Bowman SR, Smith T, Flower RJ, Bolton C (2007) Glutamate-stimulated peroxynitrite production in a brain-derived endothelial cell line is dependent on N-methyl-d-aspartate (NMDA) receptor activation. Biochem Pharmacol 73(2):228–236
Gursoy-Ozdemir Y, Can A, Dalkara T (2004) Reperfusion-induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke 35(6):1449–1453
Freeman LR, Keller JN (2012) Oxidative stress and cerebral endothelial cells: regulation of the blood–brain-barrier and antioxidant based interventions. Biochim Biophys Acta 1822(5):822–829
Gorgulu A, Kins T, Cobanoglu S, Unal F, Izgi NI, Yanik B, Kucuk M (2000) Reduction of edema and infarction by Memantine and MK-801 after focal cerebral ischaemia and reperfusion in rat. Acta Neurochir 142(11):1287–1292
Paul C, Bolton C (2002) Modulation of blood–brain barrier dysfunction and neurological deficits during acute experimental allergic encephalomyelitis by the N-methyl-d-aspartate receptor antagonist memantine. J Pharmacol Exp Ther 302(1):50–57
Acknowledgements
This study was supported by grants from the National Cheng Kung University Hospital Medical Center (Grant NCKUH-10005022 and NCKUH-10102001). The authors thank Li-Ching Lin and Lung Yu for technical support. The authors are also grateful to Ming-Tai Yu, and Ya-Chun Hsiao for services on image acquisition and analysis from the FACS-like Tissue Cytometry in the Center of Clinical Medicine, National Cheng Kung University Hospital. The authors also thank Jia-Ling Wu and Liang-Yi Wang of the Biostatistics Consulting Center, National Cheng Kung University Hospital, for statistical consulting services.
Conflicts of interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
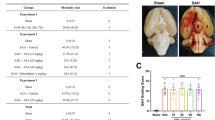
Memantine attenuated cellular apoptosis after SAH in a dose-dependent manner. The dosage of memantine that improved cellular apoptosis in SAH animals, 1 day after SAH, was investigated. Data are mean ± SEM from sham animals, vehicle-treated animals and animals treated with different dosage of memantine. Administration of 10 mg/kg memantine failed to reduce cellular apoptosis after SAH. 20mg/kg and 30 mg/kg loading of memantine led to a statistically significant protection of brain after SAH (P<0.05). (GIF 29 kb)
Rights and permissions
About this article
Cite this article
Huang, CY., Wang, LC., Wang, HK. et al. Memantine Alleviates Brain Injury and Neurobehavioral Deficits after Experimental Subarachnoid Hemorrhage. Mol Neurobiol 51, 1038–1052 (2015). https://doi.org/10.1007/s12035-014-8767-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8767-9