Abstract
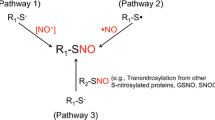
S-Nitrosylation, a redox-mediated posttranslational modification, is a result of the covalent binding nitric oxide (NO)-related species to cysteine residues of target proteins with the formation of nitrosothiols (SNOs). Normally, protein S-nitrosylation could be a cellular signaling mechanism, as is often a reversible and selective process, akin to protein phosphorylation. Emerging evidences have certified that the occurrence of aberrant S-nitrosylation of protein reactions could lead to protein misfolding, mitochondrial fission, synaptic damage, or apoptosis, thus contributing to the pathogenesis of Alzheimer’s disease (AD). In this review, we summarize the recent findings of key S-nitrosylated proteins which play crucial roles in the pathogenesis of AD and discuss how SNO proteins affect the progression of AD. In addition, it has been demonstrated that interference of S-nitrosylation could potentially protect from mitochondrial dysfunction, synaptic loss, or neuronal cell death in AD animal models. Hence, we also present the recent advances and challenges in targeting S-nitrosylated proteins for AD therapies.


Similar content being viewed by others
References
WHO, Alzheimer’s Disease International (2012) Dementia: a public health priority. World Health Organization, Geneva
Nakamura T, Tu S, Akhtar MW, Sunico CR, Okamoto S, Lipton SA (2013) Aberrant protein S-nitrosylation in neurodegenerative diseases. Neuron 78(4):596–614. doi:10.1016/j.neuron.2013.05.005
Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA (2009) S-Nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 324(5923):102–105. doi:10.1126/science.1171091
Qu J, Nakamura T, Cao G, Holland EA, McKercher SR, Lipton SA (2011) S-Nitrosylation activates Cdk5 and contributes to synaptic spine loss induced by beta-amyloid peptide. Proc Natl Acad Sci U S A 108(34):14330–14335. doi:10.1073/pnas.1105172108
Ho GP, Selvakumar B, Mukai J, Hester LD, Wang Y, Gogos JA, Snyder SH (2011) S-Nitrosylation and S-palmitoylation reciprocally regulate synaptic targeting of PSD-95. Neuron 71(1):131–141. doi:10.1016/j.neuron.2011.05.033
Forstermann U, Schmidt HH, Pollock JS, Sheng H, Mitchell JA, Warner TD, Nakane M, Murad F (1991) Isoforms of nitric oxide synthase. Characterization and purification from different cell types. Biochem Pharmacol 42(10):1849–1857
Bredt DS, Hwang PM, Glatt CE, Lowenstein C, Reed RR, Snyder SH (1991) Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature 351(6329):714–718. doi:10.1038/351714a0
Fukui H, Moraes CT (2008) The mitochondrial impairment, oxidative stress and neurodegeneration connection: reality or just an attractive hypothesis? Trends Neurosci 31(5):251–256. doi:10.1016/j.tins.2008.02.008
Canzoniero LM, Granzotto A, Turetsky DM, Choi DW, Dugan LL, Sensi SL (2013) nNOS (+) striatal neurons, a subpopulation spared in Huntington's disease, possess functional NMDA receptors but fail to generate mitochondrial ROS in response to an excitotoxic challenge. Front Physiol 4:112
Jin P, Kim JA, Choi DY, Lee YJ, Jung HS, Hong JT (2013) Anti-inflammatory and anti-amyloidogenic effects of a small molecule, 2,4-bis(p-hydroxyphenyl)-2-butenal in Tg2576 Alzheimer's disease mice model. J Neuroinflammation 10:2. doi:10.1186/1742-2094-10-2
Wilcock DM, Lewis MR, Van Nostrand WE, Davis J, Previti ML, Gharkholonarehe N, Vitek MP, Colton CA (2008) Progression of amyloid pathology to Alzheimer's disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2. J Neurosci 28(7):1537–1545. doi:10.1523/JNEUROSCI.5066-07.2008
Shi ZQ, Sunico CR, McKercher SR, Cui J, Feng GS, Nakamura T, Lipton SA (2013) S-nitrosylated SHP-2 contributes to NMDA receptor-mediated excitotoxicity in acute ischemic stroke. Proc Natl Acad Sci U S A 110(8):3137–3142. doi:10.1073/pnas.1215501110
Nakamura T, Lipton SA (2007) S-Nitrosylation and uncompetitive/fast off-rate (UFO) drug therapy in neurodegenerative disorders of protein misfolding. Cell Death Differ 14(7):1305–1314. doi:10.1038/sj.cdd.4402138
Graves DB (2012) The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J Phys D Appl Phys 45(26):263001
Wilkins HM, Kirchhof D, Manning E, Joseph JW, Linseman DA (2013) Mitochondrial glutathione transport is a key determinant of neuronal susceptibility to oxidative and nitrosative stress. J Biol Chem 288(7):5091–5101
Forrester MT, Thompson JW, Foster MW, Nogueira L, Moseley MA, Stamler JS (2009) Proteomic analysis of S-nitrosylation and denitrosylation by resin-assisted capture. Nat Biotechnol 27(6):557–559. doi:10.1038/nbt.1545
Xue Y, Liu Z, Gao X, Jin C, Wen L, Yao X, Ren J (2010) GPS-SNO: computational prediction of protein S-nitrosylation sites with a modified GPS algorithm. PloS One 5(6):e11290. doi:10.1371/journal.pone.0011290
Cuadrado-Tejedor M, Vilarino M, Cabodevilla F, Del Rio J, Frechilla D, Perez-Mediavilla A (2011) Enhanced expression of the voltage-dependent anion channel 1 (VDAC1) in Alzheimer's disease transgenic mice: an insight into the pathogenic effects of amyloid-beta. J Alzheim Dis JAD 23(2):195–206. doi:10.3233/jad-2010-100966
Guo J, Gaffrey MJ, Su D, Liu T, Camp DG 2nd, Smith RD, Qian WJ (2014) Resin-assisted enrichment of thiols as a general strategy for proteomic profiling of cysteine-based reversible modifications. Nat Protocol 9(1):64–75. doi:10.1038/nprot.2013.161
Beigi F, Gonzalez DR, Minhas KM, Sun QA, Foster MW, Khan SA, Treuer AV, Dulce RA, Harrison RW, Saraiva RM, Premer C, Schulman IH, Stamler JS, Hare JM (2012) Dynamic denitrosylation via S-nitrosoglutathione reductase regulates cardiovascular function. Proc Natl Acad Sci U S A 109(11):4314–4319. doi:10.1073/pnas.1113319109
Sun N, Hao JR, Li XY, Yin XH, Zong YY, Zhang GY, Gao C (2013) GluR6-FasL-Trx2 mediates denitrosylation and activation of procaspase-3 in cerebral ischemia/reperfusion in rats. Cell Death Dis 4:e771. doi:10.1038/cddis.2013.299
Jeon GS, Nakamura T, Lee JS, Choi WJ, Ahn SW, Lee KW, Sung JJ, Lipton SA (2013) Potential effect of S-nitrosylated protein disulfide isomerase on mutant SOD1 aggregation and neuronal cell death in amyotrophic lateral sclerosis. Mol Neurobiol. doi:10.1007/s12035-013-8562-z
Nakamura T, Lipton SA (2013) Emerging role of protein–protein transnitrosylation in cell signaling pathways. Antioxid Redox Signal 18(3):239–249. doi:10.1089/ars.2012.4703
Odajima J, Wills ZP, Ndassa YM, Terunuma M, Kretschmannova K, Deeb TZ, Geng Y, Gawrzak S, Quadros IM, Newman J, Das M, Jecrois ME, Yu Q, Li N, Bienvenu F, Moss SJ, Greenberg ME, Marto JA, Sicinski P (2011) Cyclin E constrains Cdk5 activity to regulate synaptic plasticity and memory formation. Dev Cell 21(4):655–668. doi:10.1016/j.devcel.2011.08.009
Cheung ZH, Ip NY (2012) Cdk5: a multifaceted kinase in neurodegenerative diseases. Trends Cell Biol 22(3):169–175. doi:10.1016/j.tcb.2011.11.003
Qu J, Nakamura T, Holland EA, McKercher SR, Lipton SA (2012) S-Nitrosylation of Cdk5: potential implications in amyloid-β-related neurotoxicity in Alzheimer disease. Prion 6(4):364–370
Zhang P, Yu PC, Tsang AH, Chen Y, Fu AK, Fu WY, Chung KK, Ip NY (2010) S-Nitrosylation of cyclin-dependent kinase 5 (cdk5) regulates its kinase activity and dendrite growth during neuronal development. J Neurosci 30(43):14366–14370. doi:10.1523/JNEUROSCI.3899-10.2010
Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS (2010) Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proc Natl Acad Sci U S A 107(43):18670–18675. doi:10.1073/pnas.1006586107
Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E (2008) Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci 9(7):505–518. doi:10.1038/nrn2417
Reddy PH, Beal MF (2008) Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol Med 14(2):45–53. doi:10.1016/j.molmed.2007.12.002
Wang S, Song J, Tan M, Albers KM, Jia J (2012) Mitochondrial fission proteins in peripheral blood lymphocytes are potential biomarkers for Alzheimer's disease. Eur J Neurol 19(7):1015–1022. doi:10.1111/j.1468-1331.2012.03670.x
Wang X, Su B, H-g L, Li X, Perry G, Smith MA, Zhu X (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci 29(28):9090–9103
Nakamura T, Lipton SA (2013) Emerging role of protein-protein transnitrosylation in cell signaling pathways. Antioxid Redox Signal 18(3):239–249
Scheper W, Nijholt DA, Hoozemans JJ (2011) The unfolded protein response and proteostasis in Alzheimer disease: preferential activation of autophagy by endoplasmic reticulum stress. Autophagy 7(8):910–911
Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, Marciniak SJ (2013) Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol 12(1):105–118. doi:10.1016/s1474-4422(12)70238-7
Hatahet F, Ruddock LW (2009) Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxid Redox Signal 11(11):2807–2850
Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA (2006) S-Nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 441(7092):513–517. doi:10.1038/nature04782
Gaucher C, Boudier A, Dahboul F, Parent M, Leroy P (2013) S-Nitrosation/denitrosation in cardiovascular pathologies: facts and concepts for the rational design of S-nitrosothiols. Curr Pharm Des 19(3):458–472
Sliskovic I, Raturi A, Mutus B (2005) Characterization of the S-denitrosation activity of protein disulfide isomerase. J Biol Chem 280(10):8733–8741. doi:10.1074/jbc.M408080200
Benhar M, Thompson JW, Moseley MA, Stamler JS (2010) Identification of S-nitrosylated targets of thioredoxin using a quantitative proteomic approach. Biochemistry 49(32):6963–6969
Sato N, Morishita R (2013) Plasma abeta: a possible missing link between Alzheimer disease and diabetes. Diabetes 62(4):1005–1006. doi:10.2337/db12-1549
Ito S, Ohtsuki S, Murata S, Katsukura Y, Suzuki H, Funaki M, Tachikawa M, Terasaki T (2014) Involvement of insulin-degrading enzyme in insulin- and atrial natriuretic peptide-sensitive internalization of amyloid-beta peptide in mouse brain capillary endothelial cells. J Alzheim Dis JAD 38(1):185–200. doi:10.3233/jad-122077
Kruszelnicka O (2014) Nitric oxide vs insulin secretion, action and clearance. Diabetologia 57(1):257–258. doi:10.1007/s00125-013-3082-y
Ralat LA, Ren M, Schilling AB, Tang WJ (2009) Protective role of Cys-178 against the inactivation and oligomerization of human insulin-degrading enzyme by oxidation and nitrosylation. J Biol Chem 284(49):34005–34018. doi:10.1074/jbc.M109.030627
Arold S, Sullivan P, Bilousova T, Teng E, Miller CA, Poon WW, Vinters HV, Cornwell LB, Saing T, Cole GM, Gylys KH (2012) Apolipoprotein E level and cholesterol are associated with reduced synaptic amyloid beta in Alzheimer's disease and apoE TR mouse cortex. Acta Neuropathol 123(1):39–52. doi:10.1007/s00401-011-0892-1
Wang HF, Yu JT, Zhang W, Wang W, Liu QY, Ma XY, Ding HM, Tan L (2012) SORCS1 and APOE polymorphisms interact to confer risk for late-onset Alzheimer's disease in a Northern Han Chinese population. Brain Res 1448:111–116. doi:10.1016/j.brainres.2012.01.067
Lu RC, Wang H, Tan MS, Yu JT, Tan L (2013) TMEM106B and APOE polymorphisms interact to confer risk for late-onset Alzheimer's disease in Han Chinese. J Neural Transm (Vienna, Austria : 1996). doi:10.1007/s00702-013-1106-x
Oikawa N, Hatsuta H, Murayama S, Suzuki A, Yanagisawa K (2014) Influence of APOE genotype and the presence of Alzheimer's pathology on synaptic membrane lipids of human brains. J Neurosci Res. doi:10.1002/jnr.23341
Liu CC, Kanekiyo T, Xu H, Bu G (2013) Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9(2):106–118. doi:10.1038/nrneurol.2012.263
Abrams AJ, Farooq A, Wang G (2011) S-Nitrosylation of ApoE in Alzheimer's disease. Biochemistry 50(17):3405–3407. doi:10.1021/bi200266v
Tortosa E, Galjart N, Avila J, Sayas CL (2013) MAP1B regulates microtubule dynamics by sequestering EB1/3 in the cytosol of developing neuronal cells. EMBO J 32(9):1293–1306. doi:10.1038/emboj.2013.76
Stroissnigg H, Trancikova A, Descovich L, Fuhrmann J, Kutschera W, Kostan J, Meixner A, Nothias F, Propst F (2007) S-Nitrosylation of microtubule-associated protein 1B mediates nitric-oxide-induced axon retraction. Nat Cell Biol 9(9):1035–1045. doi:10.1038/ncb1625
Yonashiro R, Kimijima Y, Shimura T, Kawaguchi K, Fukuda T, Inatome R, Yanagi S (2012) Mitochondrial ubiquitin ligase MITOL blocks S-nitrosylated MAP1B-light chain 1-mediated mitochondrial dysfunction and neuronal cell death. Proc Natl Acad Sci U S A 109(7):2382–2387. doi:10.1073/pnas.1114985109
Prota AE, Magiera MM, Kuijpers M, Bargsten K, Frey D, Wieser M, Jaussi R, Hoogenraad CC, Kammerer RA, Janke C, Steinmetz MO (2013) Structural basis of tubulin tyrosination by tubulin tyrosine ligase. J Cell Biol 200(3):259–270. doi:10.1083/jcb.201211017
Zahid S, Khan R, Oellerich M, Ahmed N, Asif AR (2013) Differential S-nitrosylation of proteins in Alzheimer's disease. Neuroscience 256C:126–136. doi:10.1016/j.neuroscience.2013.10.026
Kamnev A, Muhar M, Preinreich M, Ammer H, Propst F (2013) Difficulties in generating specific antibodies for immunohistochemical detection of nitrosylated tubulins. PloS One 8(6):e68168. doi:10.1371/journal.pone.0068168
Sirover MA (2013) GAPDH: β-amyloid mediated iron accumulation in Alzheimer’s disease: a new paradigm for oxidative stress induction in neurodegenerative disorders. In: Pratico D et al (eds) Studies on Alzheimer's disease. Springer, New York, pp 25–40
Sengupta R, Holmgren A (2013) Thioredoxin and thioredoxin reductase in relation to reversible S-nitrosylation. Antioxid Redox Signal 18(3):259–269. doi:10.1089/ars.2012.4716
Sen N, Hara MR, Kornberg MD, Cascio MB, Bae BI, Shahani N, Thomas B, Dawson TM, Dawson VL, Snyder SH, Sawa A (2008) Nitric oxide-induced nuclear GAPDH activates p300/CBP and mediates apoptosis. Nat Cell Biol 10(7):866–873. doi:10.1038/ncb1747
Sen N, Snyder SH (2011) Neurotrophin-mediated degradation of histone methyltransferase by S-nitrosylation cascade regulates neuronal differentiation. Proc Natl Acad Sci U S A 108(50):20178–20183. doi:10.1073/pnas.1117820108
Lee SB, Kim CK, Lee KH, Ahn JY (2012) S-Nitrosylation of B23/nucleophosmin by GAPDH protects cells from the SIAH1-GAPDH death cascade. J Cell Biol 199(1):65–76. doi:10.1083/jcb.201205015
Sen N, Hara MR, Ahmad AS, Cascio MB, Kamiya A, Ehmsen JT, Agrawal N, Hester L, Dore S, Snyder SH, Sawa A (2009) GOSPEL: a neuroprotective protein that binds to GAPDH upon S-nitrosylation. Neuron 63(1):81–91. doi:10.1016/j.neuron.2009.05.024
Kornberg MD, Sen N, Hara MR, Juluri KR, Nguyen JV, Snowman AM, Law L, Hester LD, Snyder SH (2010) GAPDH mediates nitrosylation of nuclear proteins. Nat Cell Biol 12(11):1094–1100. doi:10.1038/ncb2114
Chakravarti R, Aulak KS, Fox PL, Stuehr DJ (2010) GAPDH regulates cellular heme insertion into inducible nitric oxide synthase. Proc Natl Acad Sci U S A 107(42):18004–18009. doi:10.1073/pnas.1008133107
Jia J, Arif A, Willard B, Smith JD, Stuehr DJ, Hazen SL, Fox PL (2012) Protection of extraribosomal RPL13a by GAPDH and dysregulation by S-nitrosylation. Mol Cell 47(4):656–663. doi:10.1016/j.molcel.2012.06.006
Manczak M, Reddy PH (2012) Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer's disease. Hum Mol Genet 21(23):5131–5146. doi:10.1093/hmg/dds360
Yoo BC, Fountoulakis M, Cairns N, Lubec G (2001) Changes of voltage-dependent anion-selective channel proteins VDAC1 and VDAC2 brain levels in patients with Alzheimer's disease and Down syndrome. Electrophoresis 22(1):172–179. doi:10.1002/1522-2683(200101)22:1<172::AID-ELPS172>3.0.CO;2-P
Arbel N, Shoshan-Barmatz V (2010) Voltage-dependent anion channel 1-based peptides interact with Bcl-2 to prevent antiapoptotic activity. J Biol Chem 285(9):6053–6062. doi:10.1074/jbc.M109.082990
Ferrer PE, Frederick P, Gulbis JM, Dewson G, Kluck RM (2012) Translocation of a Bak C-terminus mutant from cytosol to mitochondria to mediate cytochrome C release: implications for Bak and Bax apoptotic function. PloS One 7(3):e31510
Hyman BT, Yuan J (2012) Apoptotic and non-apoptotic roles of caspases in neuronal physiology and pathophysiology. Nat Rev Neurosci 13(6):395–406. doi:10.1038/nrn3228
Du H, Guo L, Wu X, Sosunov AA, McKhann GM, Chen JX, Yan SS (2013) Cyclophilin D deficiency rescues Abeta-impaired PKA/CREB signaling and alleviates synaptic degeneration. Biochim Biophys Acta. doi:10.1016/j.bbadis.2013.03.004
Reddy PH (2013) Amyloid beta-induced glycogen synthase kinase 3beta phosphorylated VDAC1 in Alzheimer's disease: implications for synaptic dysfunction and neuronal damage. Biochim Biophys Acta 1832(12):1913–1921. doi:10.1016/j.bbadis.2013.06.012
Manczak M, Sheiko T, Craigen WJ, Reddy PH (2013) Reduced VDAC1 protects against Alzheimer's disease, mitochondria, and synaptic deficiencies. J Alzheim Dis JAD 37(4):679–690. doi:10.3233/jad-130761
Herrera JL, Fernandez C, Diaz M, Cury D, Marin R (2011) Estradiol and tamoxifen differentially regulate a plasmalemmal voltage-dependent anion channel involved in amyloid-beta induced neurotoxicity. Steroids 76(9):840–844. doi:10.1016/j.steroids.2011.02.014
Murakami K, Shimizu T (2012) Cytoplasmic superoxide radical: a possible contributing factor to intracellular Abeta oligomerization in Alzheimer disease. Commun Integr Biol 5(3):255–258. doi:10.4161/cib.19548
Fukai T, Ushio-Fukai M (2011) Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15(6):1583–1606. doi:10.1089/ars.2011.3999
Flynn JM, Melov S (2013) SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic Biol Med 62:4–12. doi:10.1016/j.freeradbiomed.2013.05.027
Bitner BR, Perez-Torres CJ, Hu L, Inoue T, Pautler RG (2012) Improvements in a mouse model of Alzheimer's disease through SOD2 overexpression are due to functional and not structural alterations. Magn Reson Insights 5:1–6. doi:10.4137/mri.s9352
Ye N, Liu S, Lin Y, Rao P (2011) Protective effects of intraperitoneal injection of TAT-SOD against focal cerebral ischemia/reperfusion injury in rats. Life Sci 89(23–24):868–874. doi:10.1016/j.lfs.2011.09.015
Mollapour M, Neckers L (2012) Post-translational modifications of Hsp90 and their contributions to chaperone regulation. Biochim Biophys Acta 1823(3):648–655. doi:10.1016/j.bbamcr.2011.07.018
Ghosh A, Chawla-Sarkar M, Stuehr DJ (2011) Hsp90 interacts with inducible NO synthase client protein in its heme-free state and then drives heme insertion by an ATP-dependent process. FASEB J 25(6):2049–2060. doi:10.1096/fj.10-180554
Crimins JL, Pooler A, Polydoro M, Luebke JI, Spires-Jones TL (2013) The intersection of amyloid beta and tau in glutamatergic synaptic dysfunction and collapse in Alzheimer's disease. Ageing Res Rev 12(3):757–763. doi:10.1016/j.arr.2013.03.002
Thompson AD, Scaglione KM, Prensner J, Gillies AT, Chinnaiyan A, Paulson HL, Jinwal UK, Dickey CA, Gestwicki JE (2012) Analysis of the tau-associated proteome reveals that exchange of Hsp70 for Hsp90 is involved in tau degradation. ACS Chem Biol 7(10):1677–1686. doi:10.1021/cb3002599
Dou F, Netzer WJ, Tanemura K, Li F, Hartl FU, Takashima A, Gouras GK, Greengard P, Xu H (2003) Chaperones increase association of tau protein with microtubules. Proc Natl Acad Sci U S A 100(2):721–726. doi:10.1073/pnas.242720499
Koren J, Inda MC, Riolo M, Uddin M, Alonso-Sabadell R, Chiosis G (2013) Blood-brain-barrier–permeable Hsp90 inhibitor reduces soluble tau burden in a mouse model of Alzheimer's disease. Alzheimers Dement 9(4):P305–P305
Blair LJ, Nordhues BA, Hill SE, Scaglione KM, O'Leary JC 3rd, Fontaine SN, Breydo L, Zhang B, Li P, Wang L, Cotman C, Paulson HL, Muschol M, Uversky VN, Klengel T, Binder EB, Kayed R, Golde TE, Berchtold N, Dickey CA (2013) Accelerated neurodegeneration through chaperone-mediated oligomerization of tau. J Clin Investig 123(10):4158–4169. doi:10.1172/jci69003
Evans CG, Wisen S, Gestwicki JE (2006) Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1-42) aggregation in vitro. J Biol Chem 281(44):33182–33191. doi:10.1074/jbc.M606192200
Retzlaff M, Stahl M, Eberl HC, Lagleder S, Beck J, Kessler H, Buchner J (2009) Hsp90 is regulated by a switch point in the C-terminal domain. EMBO Rep 10(10):1147–1153. doi:10.1038/embor.2009.153
Moskovitz J (2014) Detection and localization of methionine sulfoxide residues of specific proteins in brain tissue. Protein Pept lett 21(1):52–55
Firouzi Z, Lari P, Rashedinia M, Ramezani M, Iranshahi M, Abnous K (2014) Proteomics screening of molecular targets of curcumin in mouse brain. Life Sci. doi:10.1016/j.lfs.2013.12.200
Xiao H, Run X, Cao X, Su Y, Sun Z, Tian C, Sun S, Liang Z (2013) Temperature control can abolish anesthesia-induced tau hyperphosphorylation and partly reverse anesthesia-induced cognitive impairment in old mice. Psychiatry Clin Neurosci 67(7):493–500. doi:10.1111/pcn.12091
Sultana R, Poon HF, Cai J, Pierce WM, Merchant M, Klein JB, Markesbery WR, Butterfield DA (2006) Identification of nitrated proteins in Alzheimer's disease brain using a redox proteomics approach. Neurobiol Dis 22(1):76–87. doi:10.1016/j.nbd.2005.10.004
Korhonen K, Pastorekova S (2012) Meningiomas: role of carbonic anhydrase II. In: Hayat MA (ed) Tumors of the central nervous system, vol 7. Springer, Dordrecht, pp 11–15
Sun MK, Alkon DL (2002) Carbonic anhydrase gating of attention: memory therapy and enhancement. Trends Pharmacol Sci 23(2):83–89
Cao Z, Zhang R, Li J, Huang H, Zhang D, Zhang J, Gao J, Chen J, Huang C (2013) X-linked inhibitor of apoptosis protein (XIAP) regulation of cyclin D1 protein expression and cancer cell anchorage-independent growth via its E3 ligase-mediated protein phosphatase 2A/c-Jun axis. J Biol Chem 288(28):20238–20247
Unsain N, Higgins JM, Parker KN, Johnstone AD, Barker PA (2013) XIAP regulates caspase activity in degenerating axons. Cell Rep 4(4):751–763. doi:10.1016/j.celrep.2013.07.015
Tsang AH, Lee YI, Ko HS, Savitt JM, Pletnikova O, Troncoso JC, Dawson VL, Dawson TM, Chung KK (2009) S-Nitrosylation of XIAP compromises neuronal survival in Parkinson's disease. Proc Natl Acad Sci U S A 106(12):4900–4905. doi:10.1073/pnas.0810595106
Nakamura T, Wang L, Wong CC, Scott FL, Eckelman BP, Han X, Tzitzilonis C, Meng F, Gu Z, Holland EA, Clemente AT, Okamoto S, Salvesen GS, Riek R, Yates JR 3rd, Lipton SA (2010) Transnitrosylation of XIAP regulates caspase-dependent neuronal cell death. Mol Cell 39(2):184–195. doi:10.1016/j.molcel.2010.07.002
Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M, Owji AA, Los MJ (2014) Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 112:24–49. doi:10.1016/j.pneurobio.2013.10.004
Kim YM, Kim JH, Kwon HM, Lee DH, Won MH, Kwon YG, Kim YM (2013) Korean Red Ginseng protects endothelial cells from serum-deprived apoptosis by regulating Bcl-2 family protein dynamics and caspase S-nitrosylation. J Ginseng Res 37(4):413–424. doi:10.5142/jgr.2013.37.413
Mannick JB, Schonhoff C, Papeta N, Ghafourifar P, Szibor M, Fang K, Gaston B (2001) S-Nitrosylation of mitochondrial caspases. J Cell Biol 154(6):1111–1116. doi:10.1083/jcb.200104008
Lai YC, Pan KT, Chang GF, Hsu CH, Khoo KH, Hung CH, Jiang YJ, Ho FM, Meng TC (2011) Nitrite-mediated S-nitrosylation of caspase-3 prevents hypoxia-induced endothelial barrier dysfunction. Circ Res 109(12):1375–1386. doi:10.1161/circresaha.111.256479
Barglow KT, Knutson CG, Wishnok JS, Tannenbaum SR, Marletta MA (2011) Site-specific and redox-controlled S-nitrosation of thioredoxin. Proc Natl Acad Sci U S A 108(35):E600–E606. doi:10.1073/pnas.1110736108
Choi YB, Tenneti L, Le DA, Ortiz J, Bai G, Chen HS, Lipton SA (2000) Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nat Neurosci 3(1):15–21. doi:10.1038/71090
Paoletti P, Bellone C, Zhou Q (2013) NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14(6):383–400. doi:10.1038/nrn3504
Takahashi H, Shin Y, Cho SJ, Zago WM, Nakamura T, Gu Z, Ma Y, Furukawa H, Liddington R, Zhang D, Tong G, Chen HS, Lipton SA (2007) Hypoxia enhances S-nitrosylation-mediated NMDA receptor inhibition via a thiol oxygen sensor motif. Neuron 53(1):53–64. doi:10.1016/j.neuron.2006.11.023
Mullard A (2012) Sting of Alzheimer's failures offset by upcoming prevention trials. Nat Rev Drug Disc 11(9):657–660. doi:10.1038/nrd3842
Karran E, Mercken M, De Strooper B (2011) The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nat Rev Drug Disc 10(9):698–712. doi:10.1038/nrd3505
Talantova M, Sanz-Blasco S, Zhang X, Xia P, Akhtar MW, Okamoto S, Dziewczapolski G, Nakamura T, Cao G, Pratt AE, Kang YJ, Tu S, Molokanova E, McKercher SR, Hires SA, Sason H, Stouffer DG, Buczynski MW, Solomon JP, Michael S, Powers ET, Kelly JW, Roberts A, Tong G, Fang-Newmeyer T, Parker J, Holland EA, Zhang D, Nakanishi N, Chen HS, Wolosker H, Wang Y, Parsons LH, Ambasudhan R, Masliah E, Heinemann SF, Pina-Crespo JC, Lipton SA (2013) Abeta induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci U S A 110(27):E2518–E2527. doi:10.1073/pnas.1306832110
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81000544, 81171209, 81371406) and Shandong Provincial Natural Science Foundation, China (ZR2010HQ004, ZR2011HZ001).
Conflicts of Interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhao, QF., Yu, JT. & Tan, L. S-Nitrosylation in Alzheimer's disease. Mol Neurobiol 51, 268–280 (2015). https://doi.org/10.1007/s12035-014-8672-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8672-2