Abstract
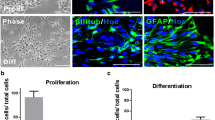
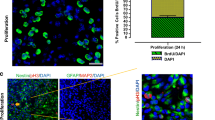
Amyloid β-peptides (Aβs) aggregate to form amyloid plaques, also known as senile plaques, which are a major pathological hallmark of Alzheimer’s disease (AD). Aβs are reported to possess proliferation effects on neural stem cells (NSCs); however, this effect remains controversial. Thus, clarification of their physiological function is an important topic. We have systematically evaluated the effects of several putative bioactive Aβs (Aβ1–40, Aβ1–42, and Aβ25–35) on NSC proliferation. Treatment of NSCs with Aβ1–42 significantly increased the number of those cells (149 ± 10 %). This was not observed with Aβ1–40 which did not have any effects on the proliferative property of NSC. Aβ25–35, on the other hand, exhibited inhibitory effects on cellular proliferation. Since cell surface glycoconjugates, such as glycolipids, glycoproteins, and proteoglycans, are known to be important for maintaining cell fate determination, including cellular proliferation, in NSCs and they undergo dramatic changes during differentiation, we examined the effect of Aβs on a number of key glycoconjugate metabolizing enzymes. Significantly, we found for the first time that Aβ1–42 altered the expression of several key glycosyltransferases and glycosidases, including fucosyltransferase IX (FUT9), sialyltransferase III (ST-III), glucosylceramide ceramidase (GLCC), and mitochondrial sialidase (Neu4). FUT9 is a key enzyme for the synthesis of the Lewis X carbohydrate epitope, which is known to be expressed in stem cells. Aβ1–42 also stimulated the Notch1 intracellular domain (NICD) by upregulation of the expression of Musashi-1 and the paired box protein, Pax6. Thus, Aβ1–42 upregulates NSC proliferation by modulating the expression of several glycogenes involved in Notch signaling.




Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- Aβ:
-
Amyloid β-protein
- FUT9:
-
Fucosyltransferase IX
- NSC:
-
Neural stem cell
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling.
- Ganglioside:
-
Recommendations (1976) IUPAC-IUB Commission on Biochemical Nomenclature (1977) The nomenclature of lipids. Lipids 12(6):455–468 and according to Svennerholm L (1963) Chromatographic separation of human brain gangliosides. J Neurochem 10:613–623
References
Selkoe DJ (1997) Alzheimer’s disease: genotypes, phenotypes, and treatments. Science 275(5300):630–631
Benilova I, Karran E, De Strooper B (2012) The toxic Abeta oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat Neurosci 15(3):349–357. doi:10.1038/nn.3028 nn.3028
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297(5580):353–356. doi:10.1126/science.1072994 297/5580/353
Itokazu Y, Kato-Negishi M, Nakatani Y, Ariga T, Yu RK (2013) Effects of amyloid beta-peptides and gangliosides on mouse neural stem cells. Neurochem Res. doi:10.1007/s11064-013-1108-y
Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB et al (1992) Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature 359(6393):322–325. doi:10.1038/359322a0
Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C et al (1992) Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature 359(6393):325–327. doi:10.1038/359325a0
Shoji M (2002) Cerebrospinal fluid Abeta40 and Abeta42: natural course and clinical usefulness. Front Biosci 7:d997–d1006
Yu RK, Nakatani Y, Yanagisawa M (2009) The role of glycosphingolipid metabolism in the developing brain. J Lipid Res 50(Suppl):S440–S445. doi:10.1194/jlr.R800028-JLR200
Yu RK, Tsai YT, Ariga T (2012) Functional roles of gangliosides in neurodevelopment: an overview of recent advances. Neurochem Res 37(6):1230–1244. doi:10.1007/s11064-012-0744-y
Ngamukote S, Yanagisawa M, Ariga T, Ando S, Yu RK (2007) Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. J Neurochem 103(6):2327–2341. doi:10.1111/j.1471-4159.2007.04910.x
Yu RK, Macala LJ, Taki T, Weinfield HM, Yu FS (1988) Developmental changes in ganglioside composition and synthesis in embryonic rat brain. J Neurochem 50(6):1825–1829
Eisenbarth GS, Walsh FS, Nirenberg M (1979) Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A 76(10):4913–4917
Kasai N, Yu RK (1983) The monoclonal antibody A2B5 is specific to ganglioside GQ1c. Brain Res 277(1):155–158
Saito M, Kitamura H, Sugiyama K (2001) The specificity of monoclonal antibody A2B5 to c-series gangliosides. J Neurochem 78(1):64–74
Majocha RE, Jungalwala FB, Rodenrys A, Marotta CA (1989) Monoclonal antibody to embryonic CNS antigen A2B5 provides evidence for the involvement of membrane components at sites of Alzheimer degeneration and detects sulfatides as well as gangliosides. J Neurochem 53(3):953–961
Freischutz B, Saito M, Rahmann H, Yu RK (1994) Activities of five different sialyltransferases in fish and rat brains. J Neurochem 62(5):1965–1973
Rosner H, Greis C, Henke-Fahle S (1988) Developmental expression in embryonic rat and chicken brain of a polysialoganglioside-antigen reacting with the monoclonal antibody Q 211. Brain Res 470(2):161–171
Raff MC, Miller RH, Noble M (1983) A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature 303(5916):390–396
Crino PB, Ullman MD, Vogt BA, Bird ED, Volicer L (1989) Brain gangliosides in dementia of the Alzheimer type. Arch Neurol 46(4):398–401
Kalanj S, Kracun I, Rosner H, Cosovic C (1991) Regional distribution of brain gangliosides in Alzheimer's disease. Neurol Croat 40(4):269–281
Kracun I, Kalanj S, Cosovic C, Talan-Hranilovic J (1990) Brain gangliosides in Alzheimer’s disease. J Hirnforsch 31(6):789–793
Kracun I, Kalanj S, Talan-Hranilovic J, Cosovic C (1992) Cortical distribution of gangliosides in Alzheimer’s disease. Neurochem Int 20(3):433–438
Svennerholm L, Gottfries CG (1994) Membrane lipids, selectively diminished in Alzheimer brains, suggest synapse loss as a primary event in early-onset form (type I) and demyelination in late-onset form (type II). J Neurochem 62(3):1039–1047
Barrier L, Ingrand S, Damjanac M, Rioux Bilan A, Hugon J, Page G (2007) Genotype-related changes of ganglioside composition in brain regions of transgenic mouse models of Alzheimer’s disease. Neurobiol Aging 28(12):1863–1872. doi:10.1016/j.neurobiolaging.2006.08.002
Ariga T, Yanagisawa M, Wakade C, Ando S, Buccafusco JJ, McDonald MP, Yu RK (2010) Ganglioside metabolism in a transgenic mouse model of Alzheimer’s disease: expression of Chol-1alpha antigens in the brain. ASN Neuro 2(4):e00044. doi:10.1042/AN20100021
Cazzaniga E, Bulbarelli A, Cassetti A, Lonati E, Re F, Palestini P, Mutoh T, Masserini M (2007) Beta-amyloid (25–35) enhances lipid metabolism and protein ubiquitination in cultured neurons. J Neurosci Res 85(10):2253–2261. doi:10.1002/jnr.21354
Copani A, Melchiorri D, Caricasole A, Martini F, Sale P, Carnevale R, Gradini R, Sortino MA, Lenti L, De Maria R, Nicoletti F (2002) Beta-amyloid-induced synthesis of the ganglioside GD3 is a requisite for cell cycle reactivation and apoptosis in neurons. J Neurosci 22(10):3963–3968
Yanagisawa M, Yu RK (2007) The expression and functions of glycoconjugates in neural stem cells. Glycobiology 17(7):57R–74R. doi:10.1093/glycob/cwm018
Kudo T, Ikehara Y, Togayachi A, Kaneko M, Hiraga T, Sasaki K, Narimatsu H (1998) Expression cloning and characterization of a novel murine alpha1, 3-fucosyltransferase, mFuc-TIX, that synthesizes the Lewis x (CD15) epitope in brain and kidney. J Biol Chem 273(41):26729–26738
Yagi H, Saito T, Yanagisawa M, Yu RK, Kato K (2012) Lewis X-carrying N-glycans regulate the proliferation of mouse embryonic neural stem cells via the Notch signaling pathway. J Biol Chem 287(29):24356–24364. doi:10.1074/jbc.M112.365643
Nakatani Y, Yanagisawa M, Suzuki Y, Yu RK (2010) Characterization of GD3 ganglioside as a novel biomarker of mouse neural stem cells. Glycobiology 20(1):78–86. doi:10.1093/glycob/cwp149
Kanemura Y, Mori H, Kobayashi S, Islam O, Kodama E, Yamamoto A, Nakanishi Y, Arita N, Yamasaki M, Okano H, Hara M, Miyake J (2002) Evaluation of in vitro proliferative activity of human fetal neural stem/progenitor cells using indirect measurements of viable cells based on cellular metabolic activity. J Neurosci Res 69(6):869–879. doi:10.1002/jnr.10377
Yanagisawa M, Yu RK (2009) O-linked beta-N-acetylglucosaminylation in mouse embryonic neural precursor cells. J Neurosci Res 87(16):3535–3545. doi:10.1002/jnr.22170
Chen Y, Dong C (2009) Abeta40 promotes neuronal cell fate in neural progenitor cells. Cell Death Differ 16(3):386–394. doi:10.1038/cdd.2008.94
Heo C, Chang KA, Choi HS, Kim HS, Kim S, Liew H, Kim JA, Yu E, Ma J, Suh YH (2007) Effects of the monomeric, oligomeric, and fibrillar Abeta42 peptides on the proliferation and differentiation of adult neural stem cells from subventricular zone. J Neurochem 102(2):493–500. doi:10.1111/j.1471-4159.2007.04499.x
Lopez-Toledano MA, Shelanski ML (2004) Neurogenic effect of beta-amyloid peptide in the development of neural stem cells. J Neurosci 24(23):5439–5444. doi:10.1523/JNEUROSCI.0974-04.2004 24/23/5439
Sotthibundhu A, Li QX, Thangnipon W, Coulson EJ (2009) Abeta(1–42) stimulates adult SVZ neurogenesis through the p75 neurotrophin receptor. Neurobiol Aging 30(12):1975–1985. doi:10.1016/j.neurobiolaging.2008.02.004
Yanagisawa M, Ariga T, Yu RK (2010) Cytotoxic effects of G(M1) ganglioside and amyloid beta-peptide on mouse embryonic neural stem cells. ASN Neuro 2(1):e00029. doi:10.1042/AN20090063
Yagi H, Yanagisawa M, Kato K, Yu RK (2010) Lysosome-associated membrane protein 1 is a major SSEA-1-carrier protein in mouse neural stem cells. Glycobiology 20(8):976–981. doi:10.1093/glycob/cwq054
Svennerholm L (1963) Chromatographic separation of human brain gangliosides. J Neurochem 10:613–623
Recommendations (1976) IUPAC-IUB Commission on Biochemical Nomenclature (1977) The nomenclature of lipids. Lipids 12(6):455–468
Sansom SN, Griffiths DS, Faedo A, Kleinjan DJ, Ruan Y, Smith J, van Heyningen V, Rubenstein JL, Livesey FJ (2009) The level of the transcription factor Pax6 is essential for controlling the balance between neural stem cell self-renewal and neurogenesis. PLoS Genet 5(6):e1000511. doi:10.1371/journal.pgen.1000511
Shimoda Y, Tajima Y, Osanai T, Katsume A, Kohara M, Kudo T, Narimatsu H, Takashima N, Ishii Y, Nakamura S, Osumi N, Sanai Y (2002) Pax6 controls the expression of Lewis x epitope in the embryonic forebrain by regulating alpha 1,3-fucosyltransferase IX expression. J Biol Chem 277(3):2033–2039. doi:10.1074/jbc.M108495200
Imai T, Tokunaga A, Yoshida T, Hashimoto M, Mikoshiba K, Weinmaster G, Nakafuku M, Okano H (2001) The neural RNA-binding protein Musashi1 translationally regulates mammalian numb gene expression by interacting with its mRNA. Mol Cell Biol 21(12):3888–3900. doi:10.1128/MCB.21.12.3888-3900.2001
Okano H, Kawahara H, Toriya M, Nakao K, Shibata S, Imai T (2005) Function of RNA-binding protein Musashi-1 in stem cells. Exp Cell Res 306(2):349–356. doi:10.1016/j.yexcr.2005.02.021
Selkoe DJ (1999) Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature 399(6738 Suppl):A23–A31
Roher AE, Chaney MO, Kuo YM, Webster SD, Stine WB, Haverkamp LJ, Woods AS, Cotter RJ, Tuohy JM, Krafft GA, Bonnell BS, Emmerling MR (1996) Morphology and toxicity of Abeta-(1–42) dimer derived from neuritic and vascular amyloid deposits of Alzheimer’s disease. J Biol Chem 271(34):20631–20635
Yan Y, Wang C (2006) Abeta42 is more rigid than Abeta40 at the C terminus: implications for Abeta aggregation and toxicity. J Mol Biol 364(5):853–862. doi:10.1016/j.jmb.2006.09.046
Yang M, Teplow DB (2008) Amyloid beta-protein monomer folding: free-energy surfaces reveal alloform-specific differences. J Mol Biol 384(2):450–464. doi:10.1016/j.jmb.2008.09.039
Kaminsky YG, Marlatt MW, Smith MA, Kosenko EA (2010) Subcellular and metabolic examination of amyloid-beta peptides in Alzheimer disease pathogenesis: evidence for Abeta(25–35). Exp Neurol 221(1):26–37. doi:10.1016/j.expneurol.2009.09.005
Lau TL, Ambroggio EE, Tew DJ, Cappai R, Masters CL, Fidelio GD, Barnham KJ, Separovic F (2006) Amyloid-beta peptide disruption of lipid membranes and the effect of metal ions. J Mol Biol 356(3):759–770. doi:10.1016/j.jmb.2005.11.091
Lau TL, Gehman JD, Wade JD, Perez K, Masters CL, Barnham KJ, Separovic F (2007) Membrane interactions and the effect of metal ions of the amyloidogenic fragment Abeta(25-35) in comparison to Abeta(1-42). Biochim Biophys Acta 1768(10):2400–2408. doi:10.1016/j.bbamem.2007.05.004
Mattson MP, Begley JG, Mark RJ, Furukawa K (1997) Abeta25-35 induces rapid lysis of red blood cells: contrast with Abeta1-42 and examination of underlying mechanisms. Brain Res 771(1):147–153
Varadarajan S, Kanski J, Aksenova M, Lauderback C, Butterfield DA (2001) Different mechanisms of oxidative stress and neurotoxicity for Alzheimer’s A beta(1–42) and A beta(25–35). J Am Chem Soc 123(24):5625–5631
Miller BL, Williams TD, Schoneich C (1996) Mechanism of sulfoxide formation through reaction of sulfur radical cation complexes with superoxide or hydroxide ion in oxygenated aqueous solution. J Am Chem Soc 118(45):11014–11025. doi:10.1021/Ja962032l
Pike CJ, Walencewicz-Wasserman AJ, Kosmoski J, Cribbs DH, Glabe CG, Cotman CW (1995) Structure-activity analyses of beta-amyloid peptides: contributions of the beta 25-35 region to aggregation and neurotoxicity. J Neurochem 64(1):253–265
Varadarajan S, Yatin S, Kanski J, Jahanshahi F, Butterfield DA (1999) Methionine residue 35 is important in amyloid beta-peptide-associated free radical oxidative stress. Brain Res Bull 50(2):133–141
Yu RK, Ando S (1980) Structures of some new complex gangliosides of fish brain. Adv Exp Med Biol 125:33–45
Ando S, Yu RK (1979) Isolation and characterization of two isomers of brain tetrasialogangliosides. J Biol Chem 254(23):12224–12229
Comelli EM, Amado M, Lustig SR, Paulson JC (2003) Identification and expression of Neu4, a novel murine sialidase. Gene 321:155–161
Shiozaki K, Koseki K, Yamaguchi K, Shiozaki M, Narimatsu H, Miyagi T (2009) Developmental change of sialidase neu4 expression in murine brain and its involvement in the regulation of neuronal cell differentiation. J Biol Chem 284(32):21157–21164. doi:10.1074/jbc.M109.012708
Tringali C, Cirillo F, Lamorte G, Papini N, Anastasia L, Lupo B, Silvestri I, Tettamanti G, Venerando B (2012) NEU4L sialidase overexpression promotes beta-catenin signaling in neuroblastoma cells, enhancing stem-like malignant cell growth. Int J Cancer 131(8):1768–1778. doi:10.1002/ijc.27450
Solter D, Knowles BB (1978) Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci U S A 75(11):5565–5569
Gooi HC, Feizi T, Kapadia A, Knowles BB, Solter D, Evans MJ (1981) Stage-specific embryonic antigen involves alpha 1 goes to 3 fucosylated type 2 blood group chains. Nature 292(5819):156–158
Acknowledgments
This work was supported in part by a VA Merit Review Award (1 IO1BX001388 to RKY) and NIH grants (RO1 NS26994 and RO1 NS11853 to RKY).
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Itokazu, Y., Yu, R.K. Amyloid β-Peptide 1–42 Modulates the Proliferation of Mouse Neural Stem Cells: Upregulation of Fucosyltransferase IX and Notch Signaling. Mol Neurobiol 50, 186–196 (2014). https://doi.org/10.1007/s12035-014-8634-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8634-8