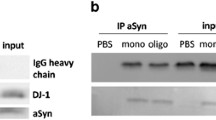
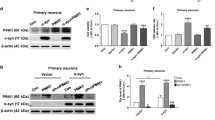
Abstract
Phosphorylation of α-synuclein (aSyn) on serine 129 is one of the major post-translation modifications found in Lewy bodies, the typical pathological hallmark of Parkinson’s disease. Here, we found that both PLK2 and PLK3 phosphorylate aSyn on serine 129 in yeast. However, only PLK2 increased aSyn cytotoxicity and the percentage of cells presenting cytoplasmic foci. Consistently, in mammalian cells, PLK2 induced aSyn phosphorylation on serine 129 and induced an increase in the size of the inclusions. Our study supports a role for PLK2 in the generation of aSyn inclusions by a mechanism that does not depend directly on serine 129 phosphorylation.



Similar content being viewed by others
References
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388(6645):839–840. doi:10.1038/42166
Gasser T, Hardy J, Mizuno Y (2011) Milestones in PD genetics. Mov Disord 26(6):1042–1048. doi:10.1002/mds.23637
Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Kruger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T (2009) Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat Genet 41(12):1308–1312. doi:10.1038/ng.487
Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, Diep L, Keim PS, Shen X, Chataway T, Schlossmacher MG, Seubert P, Schenk D, Sinha S, Gai WP, Chilcote TJ (2006) Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem 281(40):29739–29752. doi:10.1074/jbc.M600933200
Okochi M, Walter J, Koyama A, Nakajo S, Baba M, Iwatsubo T, Meijer L, Kahle PJ, Haass C (2000) Constitutive phosphorylation of the Parkinson’s disease associated alpha-synuclein. J Biol Chem 275(1):390–397
Hasegawa M, Fujiwara H, Nonaka T, Wakabayashi K, Takahashi H, Lee VM, Trojanowski JQ, Mann D, Iwatsubo T (2002) Phosphorylated alpha-synuclein is ubiquitinated in alpha-synucleinopathy lesions. J Biol Chem 277(50):49071–49076. doi:10.1074/jbc.M208046200
Paleologou KE, Schmid AW, Rospigliosi CC, Kim HY, Lamberto GR, Fredenburg RA, Lansbury PT Jr, Fernandez CO, Eliezer D, Zweckstetter M, Lashuel HA (2008) Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein. J Biol Chem 283(24):16895–16905. doi:10.1074/jbc.M800747200
Chen L, Feany MB (2005) Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci 8(5):657–663. doi:10.1038/nn1443
Freichel C, Neumann M, Ballard T, Muller V, Woolley M, Ozmen L, Borroni E, Kretzschmar HA, Haass C, Spooren W, Kahle PJ (2007) Age-dependent cognitive decline and amygdala pathology in alpha-synuclein transgenic mice. Neurobiol Aging 28(9):1421–1435. doi:10.1016/j.neurobiolaging.2006.06.013
Gorbatyuk OS, Li S, Sullivan LF, Chen W, Kondrikova G, Manfredsson FP, Mandel RJ, Muzyczka N (2008) The phosphorylation state of Ser-129 in human alpha-synuclein determines neurodegeneration in a rat model of Parkinson disease. Proc Natl Acad Sci USA 105(2):763–768. doi:10.1073/pnas.0711053105
Azeredo da Silveira S, Schneider BL, Cifuentes-Diaz C, Sage D, Abbas-Terki T, Iwatsubo T, Unser M, Aebischer P (2009) Phosphorylation does not prompt, nor prevent, the formation of alpha-synuclein toxic species in a rat model of Parkinson's disease. Hum Mol Genet 18(5):872–887. doi:10.1093/hmg/ddn417
McFarland NR, Fan Z, Xu K, Schwarzschild MA, Feany MB, Hyman BT, McLean PJ (2009) Alpha-synuclein S129 phosphorylation mutants do not alter nigrostriatal toxicity in a rat model of Parkinson disease. J Neuropathol Exp Neurol 68(5):515–524. doi:10.1097/NEN.0b013e3181a24b53
Sakamoto M, Arawaka S, Hara S, Sato H, Cui C, Machiya Y, Koyama S, Wada M, Kawanami T, Kurita K, Kato T (2009) Contribution of endogenous G-protein-coupled receptor kinases to Ser129 phosphorylation of alpha-synuclein in HEK293 cells. Biochem Biophys Res Commun 384(3):378–382. doi:10.1016/j.bbrc.2009.04.130
Nakajo S, Tsukada K, Omata K, Nakamura Y, Nakaya K (1993) A new brain-specific 14-kDa protein is a phosphoprotein. Its complete amino acid sequence and evidence for phosphorylation. Eur J Biochem 217(3):1057–1063
Inglis KJ, Chereau D, Brigham EF, Chiou SS, Schobel S, Frigon NL, Yu M, Caccavello RJ, Nelson S, Motter R, Wright S, Chian D, Santiago P, Soriano F, Ramos C, Powell K, Goldstein JM, Babcock M, Yednock T, Bard F, Basi GS, Sham H, Chilcote TJ, McConlogue L, Griswold-Prenner I, Anderson JP (2009) Polo-like kinase 2 (PLK2) phosphorylates alpha-synuclein at serine 129 in central nervous system. J Biol Chem 284(5):2598–2602. doi:10.1074/jbc.C800206200
Mbefo MK, Paleologou KE, Boucharaba A, Oueslati A, Schell H, Fournier M, Olschewski D, Yin G, Zweckstetter M, Masliah E, Kahle PJ, Hirling H, Lashuel HA (2010) Phosphorylation of synucleins by members of the Polo-like kinase family. J Biol Chem 285(4):2807–2822. doi:10.1074/jbc.M109.081950
Waxman EA, Giasson BI (2011) Characterization of kinases involved in the phosphorylation of aggregated alpha-synuclein. J Neurosci Res 89(2):231–247. doi:10.1002/jnr.22537
Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, Caldwell KA, Caldwell GA, Rochet JC, McCaffery JM, Barlowe C, Lindquist S (2008) The Parkinson's disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci USA 105(1):145–150. doi:10.1073/pnas.0710685105
Outeiro TF, Lindquist S (2003) Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science 302(5651):1772–1775. doi:10.1126/science.1090439
Tenreiro S, Outeiro TF (2010) Simple is good: yeast models of neurodegeneration. FEMS Yeast Res 10(8):970–979. doi:10.1111/j.1567-1364.2010.00649.x
Visanji NP, Wislet-Gendebien S, Oschipok LW, Zhang G, Aubert I, Fraser PE, Tandon A (2011) Effect of Ser-129 phosphorylation on interaction of alpha-synuclein with synaptic and cellular membranes. J Biol Chem 286(41):35863–35873. doi:10.1074/jbc.M111.253450
Outeiro TF, Putcha P, Tetzlaff JE, Spoelgen R, Koker M, Carvalho F, Hyman BT, McLean PJ (2008) Formation of toxic oligomeric alpha-synuclein species in living cells. PLoS One 3(4):e1867. doi:10.1371/journal.pone.0001867
McLean PJ, Kawamata H, Hyman BT (2001) Alpha-synuclein-enhanced green fluorescent protein fusion proteins form proteasome sensitive inclusions in primary neurons. Neuroscience 104(3):901–912
Engelender S, Kaminsky Z, Guo X, Sharp AH, Amaravi RK, Kleiderlein JJ, Margolis RL, Troncoso JC, Lanahan AA, Worley PF, Dawson VL, Dawson TM, Ross CA (1999) Synphilin-1 associates with alpha-synuclein and promotes the formation of cytosolic inclusions. Nat Genet 22(1):110–114. doi:10.1038/8820
Wakabayashi K, Engelender S, Yoshimoto M, Tsuji S, Ross CA, Takahashi H (2000) Synphilin-1 is present in Lewy bodies in Parkinson’s disease. Ann Neurol 47(4):521–523
Chung KK, Zhang Y, Lim KL, Tanaka Y, Huang H, Gao J, Ross CA, Dawson VL, Dawson TM (2001) Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat Med 7(10):1144–1150. doi:10.1038/nm1001-1144 nm1001-1144
Lim KL, Chew KC, Tan JM, Wang C, Chung KK, Zhang Y, Tanaka Y, Smith W, Engelender S, Ross CA, Dawson VL, Dawson TM (2005) Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J Neurosci 25(8):2002–2009. doi:10.1523/JNEUROSCI.4474-04.2005
Lee G, Tanaka M, Park K, Lee SS, Kim YM, Junn E, Lee SH, Mouradian MM (2004) Casein kinase II-mediated phosphorylation regulates alpha-synuclein/synphilin-1 interaction and inclusion body formation. J Biol Chem 279(8):6834–6839. doi:10.1074/jbc.M312760200
Avraham E, Szargel R, Eyal A, Rott R, Engelender S (2005) Glycogen synthase kinase 3beta modulates synphilin-1 ubiquitylation and cellular inclusion formation by SIAH: implications for proteasomal function and Lewy body formation. J Biol Chem 280(52):42877–42886. doi:10.1074/jbc.M505608200
Smith WW, Margolis RL, Li X, Troncoso JC, Lee MK, Dawson VL, Dawson TM, Iwatsubo T, Ross CA (2005) Alpha-synuclein phosphorylation enhances eosinophilic cytoplasmic inclusion formation in SH-SY5Y cells. J Neurosci 25(23):5544–5552. doi:10.1523/JNEUROSCI.0482-05.2005
Wang S, Xu B, Liou LC, Ren Q, Huang S, Luo Y, Zhang Z, Witt SN Alpha-synuclein disrupts stress signaling by inhibiting polo-like kinase Cdc5/Plk2. Proc Natl Acad Sci USA 109 (40):16119-16124. doi:1206286109 [pii]10.1073/pnas.1206286109
Matthew EM, Hart LS, Astrinidis A, Navaraj A, Dolloff NG, Dicker DT, Henske EP, El-Deiry WS (2009) The p53 target Plk2 interacts with TSC proteins impacting mTOR signaling, tumor growth and chemosensitivity under hypoxic conditions. Cell Cycle 8(24):4168–4175
Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC (2003) Alpha-synuclein is degraded by both autophagy and the proteasome. J Biol Chem 278(27):25009–25013. doi:10.1074/jbc.M300227200
Tanese N (1997) Small-scale density gradient sedimentation to separate and analyze multiprotein complexes. Methods 12(3):224–234. doi:10.1006/meth.1997.0475
Acknowledgments
This work was supported by Fundação para a Ciência e Tecnologia (PTDC/SAU-NEU/105215/2008 and PTDC/BIA-BCM/117975/2010) [SFRH/BPD/65890/2009 to Z.M., SFRH/BPD/35767/2007 to S.T., SFRH/BI/5177/2011 to P.A, SFRH/BD/79337/2011 to S.G]. TFO was supported by a Marie Curie International Reintegration Grant and an EMBO Installation Grant. EB is supported by EC Framework 7 Marie Curie Fellowship Training Network Grant (NEURASYNC) and by EU FP7 MEFOPA. SG was supported by AXA Research Fund.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Elisa Basso and Pedro Antas have equal contribution.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
PLK4 does not increase aSyn toxicity and foci formation. a Spotting assay of yeast cultures co-expressing aSyn (aSyn + EV) or empty vector (EV) with PLK4. The yeast cell suspensions were serial diluted (1/2) and applied as spots (4 μl) onto the surface of the solid medium either with glucose (control) or galactose (induced of aSyn and PLK4 expression) as carbon source. b Fluorescence microscopy and quantification of the number of cells presenting aSyn foci in cells expressing aSyn-GFP fusion protein alone (aSyn-GFP + EV) or together with PLK4. (JPEG 2223 kb)
Rights and permissions
About this article
Cite this article
Basso, E., Antas, P., Marijanovic, Z. et al. PLK2 Modulates α-Synuclein Aggregation in Yeast and Mammalian Cells. Mol Neurobiol 48, 854–862 (2013). https://doi.org/10.1007/s12035-013-8473-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8473-z