Abstract
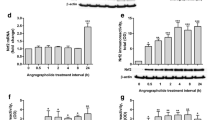
Tricyclodecan-9-yl-xanthogenate (D609) inhibits phosphatidylcholine (PC)-phospholipase C (PLC) and/or sphingomyelin (SM) synthase (SMS). Inhibiting SMS can increase ceramide levels, which can inhibit cell proliferation. Here, we examined how individual inflammatory and glia cell proliferation is altered by D609. Treatment with 100-μM D609 significantly attenuated the proliferation of RAW 264.7 macrophages, N9 and BV-2 microglia, and DITNC1 astrocytes, without affecting cell viability. D609 significantly inhibited BrdU incorporation in BV-2 microglia and caused accumulation of cells in G1 phase with decreased number of cells in the S phase. D609 treatment for 2 h significantly increased ceramide levels in BV-2 microglia, which, following a media change, returned to control levels 22 h later. This suggests that the effect of D609 may be mediated, at least in part, through ceramide increase via SMS inhibition. Western blots demonstrated that 2-h treatment of BV-2 microglia with D609 increased expression of the cyclin-dependent kinase (Cdk) inhibitor p21 and down-regulated phospho-retinoblastoma (Rb), both of which returned to basal levels 22 h after removal of D609. Exogenous C8-ceramide also inhibited BV-2 microglia proliferation without loss of viability and decreased BrdU incorporation, supporting the involvement of ceramide in D609-mediated cell cycle arrest. Our current data suggest that D609 may offer benefit after stroke (Adibhatla and Hatcher, Mol Neurobiol 41:206–217, 2010) through ceramide-mediated cell cycle arrest, thus restricting glial cell proliferation.







Similar content being viewed by others
References
Adibhatla RM, Hatcher JF, Gusain A (2012) Tricyclodecan-9-yl-xanthogenate (D609) mechanism of actions: a mini-review of literature. Neurochem Res 37:671–679
Sauer G, Amtmann E, Melber K et al (1984) DNA and RNA virus species are inhibited by xanthates, a class of antiviral compounds with unique properties. Proc Natl Acad Sci 81:3263–3267
Sultana R, Newman SF, Abdul HM et al (2006) Protective effect of D609 against amyloid-β 1-42 induced oxidative modification of neuronal proteins: redox proteomics study. J Neurosci Res 84:409–417
Zhou DH, Lauderback CM, Yu T et al (2001) D609 inhibits ionizing radiation-induced oxidative damage by acting as a potent antioxidant. J Pharmacol Exp Ther 298:103–109
Amtmann E (1996) The antiviral, antitumoural xanthate D609 is a competitive inhibitor of phosphatidylcholine-specific phospholipase C. Drugs Exp Clin Res 22:287–294
Chen C, Hu Q, Yan J et al (2007) Multiple effects of 2ME2 and D609 on the cortical expression of HIF-1α and apoptotic genes in a middle cerebral artery occlusion induced focal ischemia rat model. J Neurochem 102:1831–1841
Monick MM, Carter AB, Gudmundsson G et al (1999) A phosphatidylcholine-specific phospholipase C regulates activation of p42/44 mitogen-activated protein kinases in lipopolysaccharide-stimulated human alveolar macrophages. J Immunol 162:3005–3012
Zhang F, Zhao G, Dong Z (2001) Phosphatidylcholine-specific phospholipase C and D in stimulation of RAW264.7 mouse macrophage-like cells by lipopolysaccharide. Intl Immunopharmacol 1:1375–1384
Machleidt T, Kramer B, Adam D et al (1996) Function of the p55 TNF receptor “death domain” mediated by phosphatidylcholine-specific PLC. J Exp Med 184:725–733
Zhang L, Zhao J, Su L et al (2010) D609 inhibits progression of preexisting atheroma and promotes lesion stability in apolipoprotein E-/- mice. A role of phosphatidylcholine-specific phospholipase in atherosclerosis. Arterioscler Thromb Vasc Biol 30:411–418
Larsen EC, Hatcher JF, Adibhatla RM (2007) Effect of tricyclodecan-9-yl potassium xanthate (D609) on phospholipid metabolism and cell death during oxygen-glucose deprivation in PC12 cells. Neuroscience 146:946–961
Yu ZF, Nikolova-Karakashian M, Zhou DH et al (2000) Pivotal role for acidic sphingomyelinase in cerebral ischemia-induced ceramide and cytokine production, and neuronal apoptosis. J Mol Neurosci 15:85–97
Ruvolo PP (2001) Ceramide regulates cellular homeostasis via diverse stress signaling pathways. Leukemia 15:1153–1160
Carrasco S, Merida I (2007) Diacylglycerol, when simplicity becomes complex. Trends Biochem Sci 32:27–36
Tafesse FG, Ternes P, Holthuis JCM (2006) The multigenic sphingomyelin synthase family. J Biol Chem 281:29421–29425
Huitema K, van den Dikkenberg J, Brouwers JFHM et al (2004) Identification of a family of animal sphingomyelin synthases. EMBO J 23:33–44
Luberto C, Hannun YA (1998) SM synthase, a potential regulator of intracellular levels of ceramide and diacylglycerol during SV40 transformation. Does SM synthase account for the putative PC-specific PLC? J Biol Chem 273:14550–14559
Luberto C, Yoo DS, Suidan HS et al (2000) Differential effects of sphingomyelin hydrolysis and resynthesis on the activation of NF-kappa B in normal and SV40-transformed human fibroblasts. J Biol Chem 275:14760–14766
Riboni L, Viani P, Bassi R et al (2001) Basic fibroblast growth factor-induced proliferation of primary astrocytes. Evidence for the involvement of sphingomyelin biosynthesis. J Biol Chem 276:12797–12804
Byrnes KR, Faden AI (2007) Role of cell cycle proteins in CNS injury. Neurochem Res 32:1799–1807
Wang W, Bu B, Xie M et al (2009) Neural cell cycle dysregulation and central nervous system diseases. Prog Neurobiol 89:1–17
Ogretmen B, Hannun YA (2004) Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer 4:604–616
Adibhatla RM, Hatcher JF (2010) Protection by D609 through cell-cycle regulation after stroke. Mol Neurobiol 41:206–217
Blasi E, Barluzzi R, Bocchini V et al (1990) Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol 27:229–237
Righi M, Mori L, De Libero G et al (1989) Monokine production by microglial cell clones. Eur J Immunol 19:1443–1448
Raschke WC, Baird S, Ralph P et al (1978) Functional macrophage cell lines transformed by Abelson leukemia virus. Cell 15:261–267
Radany EH, Brenner M, Besnard F et al (1992) Directed establishment of rat brain cell lines with the phenotypic characteristics of type 1 astrocytes. Proc Natl Acad Sci 89:6467–6471
Lauderback CM, Drake J, Zhou D et al (2003) Derivatives of xanthic acid are novel antioxidants: application to synaptosomes. Free Radic Res 37:355–365
Bai A, Meier GP, Wang Y et al (2004) Prodrug modification increases potassium tricyclo[5.2.1.02,6]-decan-8-yl dithiocarbonate (D609) chemical stability and cytotoxicity against U937 leukemia cells. J Pharmacol Exp Ther 309:1051–1059
Furuya K, Ginis I, Takeda H et al (2001) Cell permeable exogenous ceramide reduces infarct size in spontaneously hypertensive rats supporting in vitro studies that have implicated ceramide in induction of tolerance to ischemia. J Cereb Blood Flow Metab 21:226–232
Yang NC, Jeng KC, Ho WM et al (2000) DHEA inhibits cell growth and induces apoptosis in BV-2 cells and the effects are inversely associated with glucose concentration in the medium. J Steroid Biochem Mol Biol 75:159–166
Wang N, Lv X, Su L et al (2006) D609 blocks cell survival and induces apoptosis in neural stem cells. Bioorg Med Chem Lett 16:4780–4783
Yakovlev AG, Faden AI (2001) Caspase-dependent apoptotic pathways in CNS injury. Mol Neurobiol 24:131–144
Chiba N, Masuda A, Yoshikai Y et al (2007) Ceramide inhibits LPS-induced production of IL-5, IL-10, and IL-13 from mast cells. J Cell Physiol 213:126–136
Bartke N, Hannun YA (2009) Bioactive sphingolipids: metabolism and function. J Lipid Res 50:S91–96
Luberto C, Kraveka JM, Hannun YA (2002) Ceramide regulation of apoptosis versus differentiation: a walk on a fine line. Lessons from neurobiology. Neurochem Res 27:609–617
Hannun YA, Obeid LM (2011) Many ceramides. J Biol Chem 286:27855–27862
Fukunaga T, Nagahama M, Hatsuzawa K et al (2000) Implication of sphingolipid metabolism in the stability of the Golgi apparatus. J Cell Sci 113(Pt 18):3299–3307
Lipsky NG, Pagano RE (1985) A vital stain for the Golgi apparatus. Science 228:745–747
Abe A, Wu D, Shayman JA et al (1992) Metabolic effects of short-chain ceramide and glucosylceramide on sphingolipids and protein kinase C. Eur J Biochem 210:765–773
Grosch S, Schiffmann S, Geisslinger G (2012) Chain length-specific properties of ceramides. Prog Lipid Res 51:50–62
Ogretmen B, Pettus BJ, Rossi MJ et al (2002) Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide in the A549 human lung adenocarcinoma cell line. Role for endogenous ceramide in mediating the action of exogenous ceramide. J Biol Chem 277:12960–12969
Takeda S, Mitsutake S, Tsuji K et al (2006) Apoptosis occurs via the ceramide recycling pathway in human HaCaT keratinocytes. J Biochem 139:255–262
Barcelo-Coblijn G, Martin ML, de Almeida RF et al (2011) Sphingomyelin and sphingomyelin synthase (SMS) in the malignant transformation of glioma cells and in 2-hydroxyoleic acid therapy. Proc Natl Acad Sci 108:19569–19574
Herrup K, Yang Y (2007) Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nat Rev Neurosci 8:368–378
Osuga H, Osuga S, Wang F et al (2000) Cyclin-dependent kinases as a therapeutic target for stroke. Proc Natl Acad Sci 97:10254–10259
Kriz J (2006) Inflammation in ischemic brain injury: timing is important. Crit Rev Neurobiol 18:145–157
Venero JL, Burguillos MA, Brundin P et al (2011) The executioners sing a new song: killer caspases activate microglia. Cell Death Differ 18:1679–1691
Lambertsen KL, Clausen BH, Babcock AA et al (2009) Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci 29:1319–1330
Lalancette-Hebert M, Gowing G, Simard A et al (2007) Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci 27:2596–2605
Ekdahl CT, Kokaia Z, Lindvall O (2009) Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience 158:1021–1029
Madinier A, Bertrand N, Mossiat C et al (2009) Microglial involvement in neuroplastic changes following focal brain ischemia in rats. PLoS ONE 4:e8101
Day TW, Wu CH, Safa AR (2009) Etoposide induces protein kinase Cdelta- and caspase-3-dependent apoptosis in neuroblastoma cancer cells. Mol Pharmacol 76:632–640
Lee S, Suk K (2007) Heme oxygenase-1 mediates cytoprotective effects of immunostimulation in microglia. Biochem Pharmacol 74:723–729
Acknowledgements
Supported by NIH R01 NS063959 and AHA 11GRNT7360066 and resources provided by Veterans Affairs Hospital, Madison, WI. The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Anchal Gusain and James F. Hatcher contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Gusain, A., Hatcher, J.F., Adibhatla, R.M. et al. Anti-proliferative Effects of Tricyclodecan-9-yl-xanthogenate (D609) Involve Ceramide and Cell Cycle Inhibition. Mol Neurobiol 45, 455–464 (2012). https://doi.org/10.1007/s12035-012-8254-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-012-8254-0