Abstract
Injury to the central nervous system (CNS) initiates a cascade of responses that is inhibitory to the regeneration of neurons and full recovery. At the site of injury, glial cells conspire with an inhibitory biochemical milieu to construct both physical and chemical barriers that prevent the outgrowth of axons to or beyond the lesion site. These inhibitors include factors derived from myelin, repulsive guidance cues, and chondroitin sulfate proteoglycans. Each bind receptors on the axon surface to initiating intracellular signaling cascades that ultimately result in cytoskeletal reorganization and growth cone collapse. Here, we present an overview of the molecules, receptors, and signaling pathways that inhibit CNS regeneration, with a particular focus on the intracellular signaling machinery that may function as convergent targets for multiple inhibitory ligands.


Similar content being viewed by others
References
David S, Aguayo AJ (1981) Axonal elongation into peripheral nervous system "bridges" after central nervous system injury in adult rats. Science 214(4523):931–933
Eftekharpour E, Karimi-Abdolrezaee S, Fehlings MG (2008) Current status of experimental cell replacement approaches to spinal cord injury. Neurosurg Focus 24(3–4):E19
Yiu G, He Z (2006) Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7(8):617–627
Carulli D et al (2005) Chondroitin sulfate proteoglycans in neural development and regeneration. Curr Opin Neurobiol 15(1):116–120
Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5(2):146–156
Fawcett JW, Asher RA (1999) The glial scar and central nervous system repair. Brain Res Bull 49(6):377–391
Gris P et al (2007) Transcriptional regulation of scar gene expression in primary astrocytes. Glia 55(11):1145–1155
Jones LL, Margolis RU, Tuszynski MH (2003) The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol 182(2):399–411
Bradbury EJ et al (2002) Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416(6881):636–640
Schmalfeldt M et al (2000) Brain derived versican V2 is a potent inhibitor of axonal growth. J Cell Sci 113(Pt 5):807–816
Ughrin YM, Chen ZJ, Levine JM (2003) Multiple regions of the NG2 proteoglycan inhibit neurite growth and induce growth cone collapse. J Neurosci 23(1):175–186
Fidler PS et al (1999) Comparing astrocytic cell lines that are inhibitory or permissive for axon growth: the major axon-inhibitory proteoglycan is NG2. J Neurosci 19(20):8778–8788
Laabs TL et al (2007) Inhibiting glycosaminoglycan chain polymerization decreases the inhibitory activity of astrocyte-derived chondroitin sulfate proteoglycans. J Neurosci 27(52):14494–14501
Kwok JC et al (2008) Proteoglycans in the central nervous system: plasticity, regeneration and their stimulation with chondroitinase ABC. Restor Neurol Neurosci 26(2–3):131–145
Crocker PR, Varki A (2001) Siglecs in the immune system. Immunology 103(2):137–145
Arquint M et al (1987) Molecular cloning and primary structure of myelin-associated glycoprotein. Proc Natl Acad Sci USA 84(2):600–604
Lai C et al (1987) Neural protein 1B236/myelin-associated glycoprotein (MAG) defines a subgroup of the immunoglobulin superfamily. Immunol Rev 100:129–151
Schachner M, Bartsch U (2000) Multiple functions of the myelin-associated glycoprotein MAG (siglec-4a) in formation and maintenance of myelin. Glia 29(2):154–165
McKerracher L et al (1994) Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron 13(4):805–811
Mukhopadhyay G et al (1994) A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron 13(3):757–767
Bartsch S et al (1997) Increased number of unmyelinated axons in optic nerves of adult mice deficient in the myelin-associated glycoprotein (MAG). Brain Res 762(1–2):231–234
Bartsch U et al (1995) Multiply myelinated axons in the optic nerve of mice deficient for the myelin-associated glycoprotein. Glia 14(2):115–122
Li C et al (1998) Myelin associated glycoprotein modulates glia–axon contact in vivo. J Neurosci Res 51(2):210–217
Lassmann H et al (1997) Dying-back oligodendrogliopathy: a late sequel of myelin-associated glycoprotein deficiency. Glia 19(2):104–110
Shen YJ et al (1998) Myelin-associated glycoprotein in myelin and expressed by Schwann cells inhibits axonal regeneration and branching. Mol Cell Neurosci 12(1–2):79–91
Bartsch U et al (1995) Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron 15(6):1375–1381
Caroni P, Schwab ME (1988) Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol 106(4):1281–1288
Caroni P, Schwab ME (1988) Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron 1(1):85–96
Bregman BS et al (1995) Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature 378(6556):498–501
Schnell L, Schwab ME (1990) Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature 343(6255):269–272
Schnell L, Schwab ME (1993) Sprouting and regeneration of lesioned corticospinal tract fibres in the adult rat spinal cord. Eur J Neurosci 5(9):1156–1171
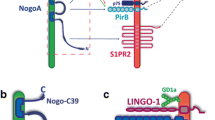
Chen MS et al (2000) Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403(6768):434–439
GrandPre T et al (2000) Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature 403(6768):439–444
Prinjha R et al (2000) Inhibitor of neurite outgrowth in humans. Nature 403(6768):383–384
Dodd DA et al (2005) Nogo-A, -B, and -C are found on the cell surface and interact together in many different cell types. J Biol Chem 280(13):12494–12502
Josephson A et al (2001) NOGO mRNA expression in adult and fetal human and rat nervous tissue and in weight drop injury. Exp Neurol 169(2):319–328
Huber AB et al (2002) Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci 22(9):3553–3567
Oertle T et al (2003) Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J Neurosci 23(13):5393–5406
Voeltz GK et al (2006) A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell 124(3):573–586
Zheng B et al (2003) Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron 38(2):213–224
Kim JE et al (2003) Axon regeneration in young adult mice lacking Nogo-A/B. Neuron 38(2):187–199
Simonen M et al (2003) Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron 38(2):201–211
Dimou L et al (2006) Nogo-A-deficient mice reveal strain-dependent differences in axonal regeneration. J Neurosci 26(21):5591–5603
Cafferty WB et al (2007) Response to correspondence: Kim et al., “Axon regeneration in young adult mice lacking Nogo-A/B.” Neuron 38, 187–199. Neuron 54(2):195–199
Cafferty WB, Strittmatter SM (2006) The Nogo–Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J Neurosci 26(47):12242–12250
Steward O et al (2007) Response to: Kim et al., “Axon regeneration in young adult mice lacking Nogo-A/B.” Neuron 38, 187–199. Neuron 54(2):191–195
Lee JK et al (2009) Reassessment of corticospinal tract regeneration in Nogo-deficient mice. J Neurosci 29(27):8649–8654
Wang KC et al (2002) Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature 417(6892):941–944
Kottis V et al (2002) Oligodendrocyte-myelin glycoprotein (OMgp) is an inhibitor of neurite outgrowth. J Neurochem 82(6):1566–1569
Mikol DD, Gulcher JR, Stefansson K (1990) The oligodendrocyte-myelin glycoprotein belongs to a distinct family of proteins and contains the HNK-1 carbohydrate. J Cell Biol 110(2):471–479
Kobe B, Deisenhofer J (1994) The leucine-rich repeat: a versatile binding motif. Trends Biochem Sci 19(10):415–421
Habib AA et al (1998) Expression of the oligodendrocyte-myelin glycoprotein by neurons in the mouse central nervous system. J Neurochem 70(4):1704–1711
Huang JK et al (2005) Glial membranes at the node of Ranvier prevent neurite outgrowth. Science 310(5755):1813–1817
Ji B et al (2008) Assessment of functional recovery and axonal sprouting in oligodendrocyte-myelin glycoprotein (OMgp) null mice after spinal cord injury. Mol Cell Neurosci 39(2):258–267
Moore SW, Tessier-Lavigne M, Kennedy TE (2007) Netrins and their receptors. Adv Exp Med Biol 621:17–31
Hedgecock EM, Culotti JG, Hall DH (1990) The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 4(1):61–85
Serafini T et al (1994) The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78(3):409–424
Manitt C et al (2001) Widespread expression of netrin-1 by neurons and oligodendrocytes in the adult mammalian spinal cord. J Neurosci 21(11):3911–3922
Low K et al (2008) Netrin-1 is a novel myelin-associated inhibitor to axon growth. J Neurosci 28(5):1099–1108
Koncina E et al (2007) Role of semaphorins during axon growth and guidance. Adv Exp Med Biol 621:50–64
Pasterkamp RJ, Verhaagen J (2006) Semaphorins in axon regeneration: developmental guidance molecules gone wrong? Philos Trans R Soc Lond B Biol Sci 361(1473):1499–1511
Luo Y, Raible D, Raper JA (1993) Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 75(2):217–227
Cohen RI et al (2003) A role for semaphorins and neuropilins in oligodendrocyte guidance. J Neurochem 85(5):1262–1278
Moreau-Fauvarque C et al (2003) The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J Neurosci 23(27):9229–9239
Pasterkamp RJ, Ruitenberg MJ, Verhaagen J (1999) Semaphorins and their receptors in olfactory axon guidance. Cell Mol Biol (Noisy-le-grand) 45(6):763–779
De Winter F, Holtmaat AJ, Verhaagen J (2002) Neuropilin and class 3 semaphorins in nervous system regeneration. Adv Exp Med Biol 515:115–139
Kaneko S et al (2006) A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med 12(12):1380–1389
Du J, Fu C, Sretavan DW (2007) Eph/ephrin signaling as a potential therapeutic target after central nervous system injury. Curr Pharm Des 13(24):2507–2518
Benson MD et al (2005) Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci USA 102(30):10694–10699
Wang KC et al (2002) P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature 420(6911):74–78
Liu BP et al (2002) Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science 297(5584):1190–1193
Fournier AE, GrandPre T, Strittmatter SM (2001) Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 409(6818):341–346
Pignot V et al (2003) Characterization of two novel proteins, NgRH1 and NgRH2, structurally and biochemically homologous to the Nogo-66 receptor. J Neurochem 85(3):717–728
Venkatesh K et al (2005) The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J Neurosci 25(4):808–822
Wong ST et al (2002) A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat Neurosci 5(12):1302–1308
Mi S et al (2004) LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci 7(3):221–228
Park JB et al (2005) A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron 45(3):345–351
Shao Z et al (2005) TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron 45(3):353–359
Fournier AE et al (2002) Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J Neurosci 22(20):8876–8883
GrandPre T, Li S, Strittmatter SM (2002) Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature 417(6888):547–551
Li S et al (2004) Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci 24(46):10511–10520
Harvey PA et al (2009) Blockade of Nogo receptor ligands promotes functional regeneration of sensory axons after dorsal root crush. J Neurosci 29(19):6285–6295
Fischer D, He Z, Benowitz LI (2004) Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J Neurosci 24(7):1646–1651
Wang X et al (2006) Delayed Nogo receptor therapy improves recovery from spinal cord contusion. Ann Neurol 60(5):540–549
Kim JE et al (2004) Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron 44(3):439–451
Zheng B et al (2005) Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc Natl Acad Sci USA 102(4):1205–1210
Chivatakarn O et al (2007) The Nogo-66 receptor NgR1 is required only for the acute growth cone-collapsing but not the chronic growth-inhibitory actions of myelin inhibitors. J Neurosci 27(27):7117–7124
McGee AW et al (2005) Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science 309(5744):2222–2226
Lee H et al (2008) Synaptic function for the Nogo-66 receptor NgR1: regulation of dendritic spine morphology and activity-dependent synaptic strength. J Neurosci 28(11):2753–2765
Atwal JK et al (2008) PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science 322(5903):967–970
Syken J et al (2006) PirB restricts ocular-dominance plasticity in visual cortex. Science 313(5794):1795–1800
Yang LJ et al (1996) Gangliosides are neuronal ligands for myelin-associated glycoprotein. Proc Natl Acad Sci USA 93(2):814–818
Vyas AA et al (2002) Gangliosides are functional nerve cell ligands for myelin-associated glycoprotein (MAG), an inhibitor of nerve regeneration. Proc Natl Acad Sci USA 99(12):8412–8417
DeBellard ME et al (1996) Myelin-associated glycoprotein inhibits axonal regeneration from a variety of neurons via interaction with a sialoglycoprotein. Mol Cell Neurosci 7(2):89–101
Mehta NR et al (2007) Gangliosides and Nogo receptors independently mediate myelin-associated glycoprotein inhibition of neurite outgrowth in different nerve cells. J Biol Chem 282(38):27875–27886
Venkatesh K et al (2007) Molecular dissection of the myelin-associated glycoprotein receptor complex reveals cell type-specific mechanisms for neurite outgrowth inhibition. J Cell Biol 177(3):393–399
Williams G et al (2008) Ganglioside inhibition of neurite outgrowth requires Nogo receptor function: identification of interaction sites and development of novel antagonists. J Biol Chem 283(24):16641–16652
Yamashita T, Higuchi H, Tohyama M (2002) The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J Cell Biol 157(4):565–570
Hu F, Strittmatter SM (2008) The N-terminal domain of Nogo-A inhibits cell adhesion and axonal outgrowth by an integrin-specific mechanism. J Neurosci 28(5):1262–1269
Goh EL et al (2008) beta1-integrin mediates myelin-associated glycoprotein signaling in neuronal growth cones. Mol Brain 1(1):10
Loschinger J et al (1997) Retinal axon growth cone responses to different environmental cues are mediated by different second-messenger systems. J Neurobiol 33(6):825–834
Song H et al (1998) Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science 281(5382):1515–1518
Hasegawa Y et al (2004) Promotion of axon regeneration by myelin-associated glycoprotein and Nogo through divergent signals downstream of Gi/G. J Neurosci 24(30):6826–6832
Sivasankaran R et al (2004) PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci 7(3):261–268
Snow DM et al (1994) Chondroitin sulfate proteoglycan elevates cytoplasmic calcium in DRG neurons. Dev Biol 166(1):87–100
Koprivica V et al (2005) EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science 310(5745):106–110
Etienne-Manneville S, Hall A (2002) Rho GTPases in cell biology. Nature 420(6916):629–635
Jalink K et al (1994) Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol 126(3):801–810
Niederost B et al (2002) Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci 22(23):10368–10376
Lehmann M et al (1999) Inactivation of Rho signaling pathway promotes CNS axon regeneration. J Neurosci 19(17):7537–7547
Fournier AE, Takizawa BT, Strittmatter SM (2003) Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci 23(4):1416–1423
Dergham P et al (2002) Rho signaling pathway targeted to promote spinal cord repair. J Neurosci 22(15):6570–6577
Domeniconi M et al (2005) MAG induces regulated intramembrane proteolysis of the p75 neurotrophin receptor to inhibit neurite outgrowth. Neuron 46(6):849–855
Yamashita T, Tohyama M (2003) The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat Neurosci 6(5):461–467
Lingor P et al (2007) Inhibition of Rho kinase (ROCK) increases neurite outgrowth on chondroitin sulphate proteoglycan in vitro and axonal regeneration in the adult optic nerve in vivo. J Neurochem 103(1):181–189
Monnier PP et al (2003) The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci 22(3):319–330
Borisoff JF et al (2003) Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol Cell Neurosci 22(3):405–416
Sahin M et al (2005) Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron 46(2):191–204
Moore SW et al (2008) Rho inhibition recruits DCC to the neuronal plasma membrane and enhances axon chemoattraction to netrin 1. Development 135(17):2855–2864
Alabed YZ et al (2006) Neuronal responses to myelin are mediated by rho kinase. J Neurochem 96(6):1616–1625
Kubo T et al (2008) Myosin IIA is required for neurite outgrowth inhibition produced by repulsive guidance molecule. J Neurochem 105(1):113–126
Hsieh SH, Ferraro GB, Fournier AE (2006) Myelin-associated inhibitors regulate cofilin phosphorylation and neuronal inhibition through LIM kinase and Slingshot phosphatase. J Neurosci 26(3):1006–1015
Bamburg JR, Bernstein BW (2008) ADF/cofilin. Curr Biol 18(7):R273–R275
Mimura F et al (2006) Myelin-associated glycoprotein inhibits microtubule assembly by a Rho-kinase-dependent mechanism. J Biol Chem 281(23):15970–15979
Alabed YZ et al (2007) Identification of CRMP4 as a convergent regulator of axon outgrowth inhibition. J Neurosci 27(7):1702–1711
Fukata Y et al (2002) CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol 4(8):583–591
Quinn CC et al (2003) TUC-4b, a novel TUC family variant, regulates neurite outgrowth and associates with vesicles in the growth cone. J Neurosci 23(7):2815–2823
Cai D et al (2001) Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci 21(13):4731–4739
Gao Y et al (2003) Neurotrophins elevate cAMP to reach a threshold required to overcome inhibition by MAG through extracellular signal-regulated kinase-dependent inhibition of phosphodiesterase. J Neurosci 23(37):11770–11777
Cai D et al (1999) Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron 22(1):89–101
Qiu J et al (2002) Spinal axon regeneration induced by elevation of cyclic AMP. Neuron 34(6):895–903
Neumann S et al (2002) Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron 34(6):885–893
Gao Y et al (2004) Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron 44(4):609–621
Cai D et al (2002) Arginase I and polyamines act downstream from cyclic AMP in overcoming inhibition of axonal growth MAG and myelin in vitro. Neuron 35(4):711–719
Cao Z et al (2006) The cytokine interleukin-6 is sufficient but not necessary to mimic the peripheral conditioning lesion effect on axonal growth. J Neurosci 26(20):5565–5573
Wen Z et al (2007) BMP gradients steer nerve growth cones by a balancing act of LIM kinase and Slingshot phosphatase on ADF/cofilin. J Cell Biol 178(1):107–119
Benowitz L, Yin Y (2008) Rewiring the injured CNS: lessons from the optic nerve. Exp Neurol 209(2):389–398
Park KK et al (2008) Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322(5903):963–966
Neumann S, Woolf CJ (1999) Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron 23(1):83–91
Seijffers R, Allchorne AJ, Woolf CJ (2006) The transcription factor ATF-3 promotes neurite outgrowth. Mol Cell Neurosci 32(1–2):143–154
Dill J et al (2008) Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS. J Neurosci 28(36):8914–8928
Bomze HM et al (2001) Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nat Neurosci 4(1):38–43
Temporin K et al (2008) IL-1beta promotes neurite outgrowth by deactivating RhoA via p38 MAPK pathway. Biochem Biophys Res Commun 365(2):375–380
Acknowledgments
We would like to thank Dr. Aaron McGee and Corrie Fox for their comments and suggestions on the manuscript and graphic work, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nash, M., Pribiag, H., Fournier, A.E. et al. Central Nervous System Regeneration Inhibitors and their Intracellular Substrates. Mol Neurobiol 40, 224–235 (2009). https://doi.org/10.1007/s12035-009-8083-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-009-8083-y