Abstract
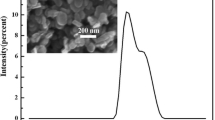
Amorphous boron powders with small particle size, narrow size distribution and high purity are very important in the high-tech fields. Mechanochemical synthesis was used to prepare amorphous boron nanoparticles. Synthesis process stage was carried out using stoichiometric amounts of B2O3 and Mg powders (6.7 g). Milling was carried out under argon atmosphere in the high-energy planetary ball mill with a ball-to-powder weight ratio (32 : 1) for 10 h. The vial rotation speed was about 440 rpm. Milled products were leached by 28% hydrochloric acid (only one) to remove impurities. Boron powders were obtained after centrifuging, decanting, washing and drying operations. Sample was characterized by inductively coupled plasma (ICP), energy-dispersive spectroscopy, X-ray diffraction, scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The ICP results showed that boron powders with purity about 91 wt% can be prepared in the planetary ball mill. Also, the leached powders had an amorphous structure. According to the SEM observation, average particle size of boron powders was smaller than 32 nm and the yield of synthesized nanoboron was more than 74%.








Similar content being viewed by others
References
Devener B V, Perez J P L, Jankovich J and Anderson S L 2009 J. Energy Fuels 3 6111
Yoo B U, Nersisyan H H, Ryu H Y, Lee J S and Lee J H 2014 Combust. Flame 16 3222
Neelameggham R 2012 J. Manuf. Sci. Prod. 12 155
Wang J, Gu Y, Li Z, Wang W and Fu Z 2013 Mater. Res. Bull. 48 2018
Tavadze G F and Shteinberg A S 2013 Production of advanced materials by methods of self-propagating high temperature synthesis (Berlin, Heidelberg: Springer)
Vignolo M, Romano G, Martinelli A, Bernini C and Siri A 2012 IEEE Trans. Appl. Supercond. 22 6200606
Shin W G, Calder S, Ugurlu O and Girshick S L 2011 J. Nanopart. Res. 13 7187
Bellott B J, Noh W, Nuzzo R G and Girolami G S 2009 J. Chem. Commun. 22 3214
Devener B V, Perez J P L and Anderson S L 2009 Mater. Res. 24 3462
Darvishi A H, Sabin B, Rene D and Julie J 2003 Process for the production of elemental boron by solid state reaction, World Intellectual Property Organization Patent, International publication number WO 03/051773
Ricceri R and Matteazzi P 2003 Powder Metall. 39 48
Agaogulliari D, Balci O and Duman I 2010 Mechanism & effects of various reducing agents on the fabrication of elemental boron. In: 19th international conference on metallurgy and materials, Roznov Pod Radhostem, Czech Republic, p 748–752
Fan G J, Song X P, Quan M X and Hu Z Q 1996 Scr. Mater. 35 1065
Ohara S, Sato K, Tan Z, Shimoda H, Ueda M and Fukui T 2010 J. Alloy Compd. 504 L17
Suryarayana C 2001 Prog. Mater. Sci. 46 1
Ciurowa K W and Gamrat K 2007 Mater. Sci–Poland 25 219
Mccormic R 2015 Mechanical alloying and mechanically induced chemical reactions. In: Handbook on the physics and chemistry of Rare Earths (eds) Jean-Claude Bnzli and Vitalij K Pecharsky (Elsevier) vol 48 p 2–379
Takacs L 2002 Prog. Mater. Sci. 47 355
Moore J J and Feng H J 1995 Prog. Mater. Sci. 39 275
Sundaram V, Logan K V and Speyer R F 1997 J. Mater Res. 12 2657
Mingliang M, Xinkuan L, Shengqi X, Donglang C and Jing’en Z 2001 J. Mater. Process. Technol. 116 124
Eckert J, Schultz L, Hellstern E and Urban K 1988 J. Appl. Phys. 64 3224
El-Eskandarany M S, Aoki K, Itoh H and Suzuki K 1991 J. Less Common Met. 169 235
Guo W, Iasonna A, Magini M, Martelli S and Padella F 1994 J. Mater. Sci. 29 2436
Dou Z H, Zhang T, Shi G Y, Peng C, Wen M and He J C 2014 J. Trans. Nonferrous Met. Soc. China 24 1446
SB Boron 86 www.sbboron.com/pdf/SBBoron86specsheet.pdf
SB Boron 90 www.sbboron.com/pdf/SBBoron90specsheet.pdf
Weimin W, Zhengyi F, Hao W and Runzhang Y 2002 J. Mater. Proc. Technol. 128 162
Yazici S and Derin B 2011 Int. J. Refractory Met. Hard Mater. 29 90
Nersisyan H H, Joo S H, Yoo B U, Cho Y H et al 2015 Combust. Flame 162 3316
Perry D L 2011 Handbook of inorganic compounds (CRC Press) 2nd ed.
Zhou J and Bai P 2015 Asia-pacific J. Chem. Eng. 10 325
Gan Y, Lim Y S and Qiao L 2012 Combust. Flame 159 1732
Acknowledgements
We thank the financial support from Malek—Ashtar University of Technology. SM thanks and appreciations also go to Eng. Eghdamtalab, Eng. Zarei and Eng. Kardan Halvaei in developing the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
SEIFOLAZADEH, A., MOHAMMADI, S. Synthesis and characterization of nanoboron powders prepared with mechanochemical reaction between B2O3 and Mg powders. Bull Mater Sci 39, 479–486 (2016). https://doi.org/10.1007/s12034-016-1150-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-016-1150-x