Abstract
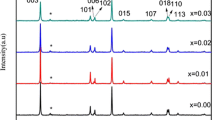
LiNiO2 and substituted nickel oxides, LiNi0·8M0·2O2 and LiCo0·8M0·2O2 (M = Mg2+, Ca2+, Ba2+), have been synthesized using simple solid state technique and used as cathode active materials for lithium rechargeable cells. Physical properties of the synthesized products are discussed in the structural (XRD, TEM, SEM with EDAX) and spectroscopic (FTIR) measurements. XRD results show that the compounds are similar to LiNiO2 in structure. TEM and SEM analyses were used to examine the particle size, nature and morphological aspects of the synthesized oxides. The composition of the materials was explored by EDAX analysis. Electrochemical studies were carried out in the range 3–4·5 V (vs Li metal) using 1 M LiBF4 in ethylene carbonate/dimethyl carbonate as the electrolyte. The doping involving 20% Mg resulted in a discharge capacity of 185 mAhg−1 at 0·1 mA/cm2 and remained stable even after 25 cycles. Discharge capacity retention for Mg doped lithium nickelate at 25th cycle was noted to be nearly 7% higher than for the undoped material.
Similar content being viewed by others
References
Arai H, Okada S, Ohtsuka H, Ichimura M and Yamaki J 1995 Solid State Ionics 80 261
Arai H, Okada S, Sakurai Y and Yamaki Y 1998 Solid State Ionics 109 295
Azaroff L V and Buerger M J 1976 The powder method (McGraw Hill)
Barrett C and Massalski T B 1968 Structure of metals (Eurasia: McGraw Hill) 3rd ed
Broussely M, Perton F, Labat J, Staniewicz R J and Romero A 1993 J. Power Sources 43–44 209
Broussely M et al 1995 J. Power Sources 54 109
Cullity B D 1978 Elements of X-ray diffraction (Addison Wesley) 2nd ed
Dahn J R, Von Sacken U, Juzkow M W and Al-Janaby H 1991 J. Electrochem. Soc. 138 2207
Dahn J R, Fuller E W, Obrovac M and Von Sacken U 1994 Solid State Ionics 69 265
Hasegawa M, Bito Y, Ito S, Murata T and Toyoguchi Y 1997 U.S. Patent 5,631,105
Huang W W and Frech R 1996 Solid State Ionics 86–88 395
Julien C, Nazri G A and Rougier A 2000 Solid State Ionics 135 121
Kanno R, Kubo H, Kawamoto Y, Kamiyama T, Izumi F, Takeda Y and Takano M 1994 J. Solid State Chem. 110 216
Klug H P and Alexander L E 1967 X-ray diffraction procedure (John Wiley)
Kumta P N, Chang C C and Sriram M A 2000 U.S. Patent 6,017,654
Mohan Rao M, Liebenow C, Jayalakshmi M, Wulff H, Guth U and Scholz F 2001 J. Solid State Electrochem. 5 348
Moore R K and White W B 1970 J. Am. Ceram. Soc. 53 679
Morales J, Vicente C P and Tirado J L 1990 Mater. Res. Bull. 25 623
Ohzuku T and Ueda A 1994 Solid State Ionics 69 201
Ohzuku T, Ueda A and Kouguchi M 1995 J. Electrochem. Soc. 142 4033
Peres J P, Delmas C, Rougier A, Broussely M, Perton F, Biensan P and Willmann P 1996 J. Phys. Chem. Solids 57 1057
Pouillerie C, Croguennec L, Biensan Ph, Willmann P and Delmas C 2000 J. Electrochem. Soc. 147 2061
Prabakaran S R S, Michael M S, Radhakrishnan S and Julien C 1997 J. Mater. Chem. 7 1791
Preudhomme J and Tarte P 1972 Spectrochim. Acta A28 69
Rougier A, Gravereau P and Delmas C 1996 J. Electrochem. Soc. 143 1168
Sathiyamoorthi R and Vasudevan T 2007a Electrochem. Commun. 9 416
Sathiyamoorthi R and Vasudevan T 2007b Mater. Res. Bull. (in press)
Takanishi K, Matsuda Y and Tsukamoto J 1997 U.S. Patent 5,679,481
Tarte P and Preudhomme J 1970 J. Spectrochim. Acta A26 747
Thomas M G S R, David W I F, Goodenough J B and Groves P 1985 Mater. Res. Bull. 20 1137
Zhecheva E and Stoyanova R 1993 Solid State Ionics 66 143
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sathiyamoorthi, R., Manisankar, P., Shakkthivel, P. et al. Synthesis, characterization and electrochemical studies of LiNi0·8M0·2O2 cathode material for rechargeable lithium batteries. Bull Mater Sci 31, 441–447 (2008). https://doi.org/10.1007/s12034-008-0069-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-008-0069-2