Abstract
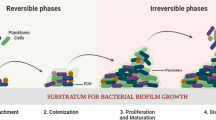
Antibiotics have been denoted as the orthodox therapeutic agents for fighting bacteria-related infections in clinical practices for decades. Nevertheless, overuse of antibiotics has led to the upsurge of species with antimicrobial resistance (AMR) or multi-drug resistance. Bacteria can also grow into the biofilm, which accounts for at least two-thirds of infections. Distinct gene expression and self-produced heterogeneous hydrated extracellular polymeric substance matrix architecture of biofilm contribute to their tolerance and externally manifest as antibiotic resistance. In this review, the difficulties in combating biofilm formation and AMR are introduced, and novel alternatives to antibiotics such as metal nanoparticles and quaternary ammonium compounds, chitosan and its derivatives, antimicrobial peptides, stimuli-responsive materials, phage therapy and other therapeutic strategies, from compounds to hydrogel, from inorganic to biological, are discussed. We expect to provide useful information for the readers who are seeking for solutions to the problem of AMR and biofilm-related infections.









Similar content being viewed by others
References
Hall, C. W., & Mah, T. F. (2017). Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiology Reviews, 41(3), 276–301.
Blair, J. M. A., Webber, M. A., Baylay, A. J., Ogbolu, D. O., & Piddock, L. J. V. (2015). Molecular mechanisms of antibiotic resistance. Nature Reviews Microbiology, 13(1), 42–51.
Rodríguez-Rojas, A., Rodríguez-Beltrán, J., Couce, A., & Blázquez, J. (2013). Antibiotics and antibiotic resistance: A bitter fight against evolution. International Journal of Medical Microbiology, 303(6–7), 293–297.
Rice, L. B. (2008). Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. Journal of Infectious Diseases, 197(8), 1079–1081.
Dwivedi, P., Narvi, S. S., & Tewari, R. P. (2013). Application of polymer nanocomposites in the nanomedicine landscape: Envisaging strategies to combat implant associated infections. Journal of Applied Biomaterials & Functional Materials, 11(3), 129–142.
Rosa, T. F., et al. (2020). Alternatives for the treatment of infections caused by ESKAPE pathogens. Journal of Clinical Pharmacy and Therapeutics, 45(4), 863–873.
Roca, I., et al. (2015). The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infections, 6, 22–29.
Hall-Stoodley, L., Costerton, J. W., & Stoodley, P. (2004). Bacterial biofilms: From the natural environment to infectious diseases. Nature Reviews Microbiology, 2(5), 95–108.
Rmling, U., & Balsalobre, C. (2012). Biofilm infections, their resilience to therapy and innovative treatment strategies. Journal of Internal Medicine, 272(6), 541–561.
Kostakioti, M., Hadjifrangiskou, M., & Hultgren, S. J. (2013). Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harbor Perspectives in Medicine, 3(4), a010306.
Valentini, M., Gonzalez, D., Mavridou, D. A., & Filoux, A. (2018). Lifestyle transitions and adaptive pathogenesis of Pseudomonas aeruginosa. Current Opinion in Microbiology, 41, 15–20.
Lister, J. L., & Horswill, A. R. (2014). Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Frontiers in Cellular and Infection Microbiology, 4, 178.
Beloin, C., & Ghigo, J. M. (2008). Escherichia coli biofilms. Current Topics in Microbiology and Immunology, 322, 249–289.
Flemming, H. C., & Wingender, J. (2010). The biofilm matrix. Nature Reviews Microbiology, 8, 623–633.
Fong, J. N. C., & Yildiz, F. H. (2015). Biofilm matrix proteins. Microbiology Spectrum. https://doi.org/10.1128/microbiolspec.MB-0004-2014
Limoli, D. H., Jones, C. J., & Wozniak, D. J. (2015). Bacterial extracellular polysaccharides in biofilm formation and function. Microbiology Spectrum. https://doi.org/10.1128/microbiolspec.MB-0011-2014
Werner, E., et al. (2004). Stratified growth in Pseudomonas aeruginosa biofilms. Applied and Environment Microbiology, 70(10), 6188–6196.
Stewart, P. S., & Franklin, M. J. (2008). Physiological heterogeneity in biofilms. Nature Reviews Microbiology, 6(3), 199.
Campoccia, D., Mirzaei, R., Montanaro, L., & Arciola, C. R. (2019). Hijacking of immune defences by biofilms: A multifront strategy. Biofouling, 35(10), 1055–1074.
Taylor, P. K., Yeung, A., & Hancock, R. (2014). Antibiotic resistance in Pseudomonas aeruginosa biofilms: Towards the development of novel anti-biofilm therapies. Journal of Biotechnology, 191, 121–130.
Kim, J. S., et al. (2011). Bacterial persisters tolerate antibiotics by not producing hydroxyl radicals. Biochemical and Biophysical Research Communications, 413(1), 105–110.
Davies, D. (2003). Understanding biofilm resistance to antibacterial agents. Nature Reviews. Drug Discovery, 2(2), 114–122.
Costerton, J. W., Lewandowski, Z., Caldwell, D. E., Korber, D. R., & Lappinscott, H. M. (1995). Microbial biofilms. Annual Review of Microbiology, 49, 711–745.
Costerton, J. W., Stewart, P. S., & Greenberg, E. P. (1999). Bacterial biofilms: A common cause of persistent infections. Science, 284(5418), 1318–1322.
Gordon, R., Pedro, M. J., & Luis, L. (2010). Candida biofilms on implanted biomaterials: A clinically significant problem. FEMS Yeast Research, 7, 979–986.
Kojic, E. M., & Darouiche, R. O. (2004). Candida infections of medical devices. Clinical Microbiology Reviews, 17(2), 255–267.
Olaya, R., & Jean-Marc, G. (2012). Multi-species biofilms: How to avoid unfriendly neighbors. FEMS Microbiology Reviews, 36(5), 972–989.
Whiteley, M., et al. (2001). Gene expression in Pseudomonas aeruginosa biofilms. Nature, 413(6858), 860–864.
Khan, F., Lee, J., Manivasagan, P., Pham, D. T. N., Oh, J., & Kim, Y. (2019). Synthesis and characterization of chitosan oligosaccharide-capped gold nanoparticles as an effective antibiofilm drug against the Pseudomonas aeruginosa PAO1. Microbial Pathogenesis, 135, 103623.
Toutain, C. M., Caizza, N. C., Zegans, M. E., & O’Toole, G. A. (2007). Roles for flagellar stators in biofilm formation by Pseudomonas aeruginosa. Research in Microbiology, 158(5), 471–477.
Caiazza, N. C., Merritt, J. H., Brothers, K. M., & O’Toole, G. A. (2007). Inverse regulation of biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. Journal of Bacteriology, 189(9), 3603–3612.
Barken, K. B., et al. (2008). Roles of type IV pili, flagellum-mediated motility and extracellular DNA in the formation of mature multicellular structures in Pseudomonas aeruginosa biofilms. Environmental Microbiology, 10(9), 2331–2343.
Petrova, O. E., & Sauer, K. (2016). Escaping the biofilm in more than one way: Desorption, detachment or dispersion. Current Opinion in Microbiology, 30, 67–78.
Brackman, G., & Coenye, T. (2015). Quorum sensing inhibitors as anti-biofilm agents. Current Pharmaceutical Design, 21(1), 5–11.
Zgurskaya, H. I., & Rybenkov, V. V. (2020). Permeability barriers of Gram-negative pathogens. Annals of the New York Academy of Sciences, 1459, 5–18.
Rodríguez-Martínez, J. M., Poirel, L., & Nordmann, P. (2009). Molecular epidemiology and mechanisms of carbapenem resistance in Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy, 53(11), 4783–4788.
Trias, J., & Nikaido, H. (1990). Outer membrane protein D2 catalyzes facilitated diffusion of carbapenems and penems through the outer membrane of Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy, 34(1), 52–57.
Routh, M. D., Zalucki, Y., Su, C. C., Zhang, Q., Shafer, W. M., & Yu, E. W. (2011). Efflux pumps of the resistance-nodulation-division family: A perspective of their structure, function and regulation in Gram-negative bacteria. Advances in Enzymology and Related Areas of Molecular Biology, 77, 109–146.
Yang, H. B., Hou, W. T., Cheng, M. T., Jiang, Y. L., Chen, Y., & Zhou, C. Z. (2018). Structure of a MacAB-like efflux pump from Streptococcus pneumoniae. Nature Communications, 9(1), 196.
Chambers, C. S., et al. (2019). Defying multidrug resistance! modulation of related transporters by flavonoids and flavonolignans. Journal of Agricultural and Food Chemistry, 68(7), 1763–1769.
Brooun, A., Liu, S., & Lewis, K. (2000). A dose-response study of antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrobial Agents and Chemotherapy, 44(3), 640–646.
Paul, M., Yahav, D., Bivas, A., Fraser, A., & Leibovici, L. (2010). Anti-pseudomonal beta-lactams for the initial, empirical, treatment of febrile neutropenia: Comparison of beta-lactams. Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.CD005197.pub3
Helfand, M. S., & Bonomo, R. A. (2003). β-lactamases: A survey of protein diversity. Current Drug Targets: Infectious Disorders, 3(1), 9–23.
Lodge, J. M., Minchin, S. D., Piddock, L., & Busby, S. J. (1990). Cloning, sequencing and analysis of the structural gene and regulatory region of the Pseudomonas aeruginosa chromosomal ampC β-lactamase. The Biochemical Journal, 272(3), 627–631.
Poole, K. (2004). Resistance to β-lactam antibiotics. Cellular and Molecular Life Sciences, 61(17), 2200–2223.
Jacoby, G. (2009). AmpC β-lactamases. Clinical Microbiology Reviews, 22(1), 161–182.
Livermore, D. M. (1992). Interplay of impermeability and chromosomal beta-lactamase activity in imipenem-resistant Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy, 36(9), 2046–2048.
Nguyen, D., et al. (2011). Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science, 334(6058), 982–986.
Poole, K. (2012). Bacterial stress responses as determinants of antimicrobial resistance. Journal of Antimicrobial Chemotherapy, 67(9), 2069–2089.
Dalebroux, Z. D., Svensson, S. L., Gaynor, E. C., & Swanson, M. S. (2010). ppGpp conjures bacterial virulence. Microbiology and Molecular Biology Reviews, 74(2), 171–199.
Potrykus, K., & Cashel, M. (2008). (p)ppGpp: Still magical? Annual Review of Microbiology, 62, 35–51.
Hauryliuk, V., Atkinson, G. C., Murakami, K. S., Tenson, T., & Gerdes, K. (2015). Recent functional insights into the role of (p) ppGpp in bacterial physiology. Nature Reviews Microbiology, 13(5), 298–309.
Kriel, A., et al. (2012). Direct regulation of GTP homeostasis by (p)ppGpp: A critical component of viability and stress resistance. Molecular Cell, 48(2), 231–241.
Kalia, D., et al. (2013). Nucleotide, c-di-GMP, c-di-AMP, cGMP, cAMP, (p)ppGpp signaling in bacteria and implications in pathogenesis. Chemical Society Reviews, 42(1), 305–341.
Jenal, U., Reinders, A., & Lori, C. (2017). Cyclic di-GMP: Second messenger extraordinaire. Nature Reviews Microbiology, 15(5), 271–284.
Skotnicka, D., Petters, T., Heering, J., Hoppert, M., Kaever, V., & Søgaard-Andersen, L. (2016). Cyclic di-GMP regulates type IV pilus-dependent motility in Myxococcus xanthus. Journal of Bacteriology, 198(1), 77–90.
Kazmierczak, B. I., Lebron, M. B., & Murray, T. S. (2006). Analysis of FimX, a phosphodiesterase that governs twitching motility in Pseudomonas aeruginosa. Molecular Microbiology, 60(4), 1026–1043.
Jones, C. J., et al. (2015). C-di-GMP regulates motile to sessile transition by modulating MshA pili biogenesis and near-surface motility behavior in Vibrio cholerae. PLoS Pathogens, 11(10), e1005068.
Serra, D. O., Richter, A. M., Klauck, G., Mika, F., & Hengge, R. (2013). Microanatomy at cellular resolution and spatial order of physiological differentiation in a bacterial biofilm. MBio, 4(2), e00103-13.
Valentini, M., & Filloux, A. (2016). Biofilms and cyclic di-GMP (c-di-GMP) signaling: Lessons from Pseudomonas aeruginosa and other bacteria. Journal of Biological Chemistry, 291(24), 12547–12555.
Waters, C. M., & Bassler, B. L. (2005). Quorum sensing: Cell-to-cell communication in bacteria. Annual Review of Cell and Developmental Biology, 21(1), 319–346.
Mukherjee, S., & Bassler, B. L. (2019). Bacterial quorum sensing in complex and dynamically changing environments. Nature Reviews Microbiology, 17(6), 371–382.
Bjarnsholt, T., et al. (2005). Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology, 151, 373–383.
Yarwood, J. M., Bartels, D. J., Volper, E. M., & Greenberg, E. P. (2004). Quorum sensing in Staphylococcus aureus biofilms. Journal of Bacteriology, 186(6), 1838–1850.
Deng, W., et al. (2017). Assembly, structure, function and regulation of type III secretion systems. Nature Reviews Microbiology, 15(6), 323–337.
Defoirdt, T. (2017). Quorum-sensing systems as targets for antivirulence therapy. Trends in Microbiology, 26(4), 313–328.
Mah, T. F., Pitts, B., Pellock, B., Walker, G. C., Stewart, P. S., & O’Toole, G. A. (2003). A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature, 426(6964), 306–310.
Sadovskaya, I., Vinogradov, E., Li, J., Hachani, A., Kowalska, K., & Filloux, A. (2010). High-level antibiotic resistance in Pseudomonas aeruginosa biofilm: The ndvB gene is involved in the production of highly glycerol-phosphorylatedβ-(1→3)-glucans, which bind aminoglycosides. Glycobiology, 7, 895–904.
Southey-Pillig, C. J., Davies, D. G., & Sauer, K. (2005). Characterization of temporal protein production in Pseudomonas aeruginosa biofilms. Journal of Bacteriology, 187(23), 8114–8126.
Liao, J., & Sauer, K. (2012). The MerR-like transcriptional regulator BrlR contributes to Pseudomonas aeruginosa biofilm tolerance. Journal of Bacteriology, 194(18), 4823–4836.
Sirelkhatim, A., et al. (2015). Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nanomicro Letters, 7(3), 219–242.
Webster, T. J., & Seil, J. T. (2012). Antimicrobial applications of nanotechnology: Methods and literature. International Journal of Nanomedicine, 7, 2767–2781.
Alavi, M., & Karimi, N. (2019). Biosynthesis of Ag and Cu NPs by secondary metabolites of usnic acid and thymol with biological macromolecules aggregation and antibacterial activities against multi drug resistant (MDR) bacteria. International Journal of Biological Macromolecules, 128, 893–901.
Wang, X., Han, Q., Yu, N., Wang, T., Wang, C., & Yang, R. (2018). GO-AgCl/Ag nanocomposites with enhanced visible light-driven catalytic properties for antibacterial and biofilm-disrupting applications. Colloids and Surfaces. B, Biointerfaces, 162, 296–305.
Zhang, L., Jiang, Y., Ding, Y., Povey, M., & York, D. (2007). Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). Journal of Nanoparticle Research, 9(3), 479–489.
Brayner, R., Ferrari-Iliou, R., Brivois, N., Djediat, S., Benedetti, M. F., & Fiévet, F. (2006). Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Letters, 6(4), 866–870.
Sawai, J., Shoji, S., Igarashi, H., Hashimoto, A., Kokugan, T., Shimizu, M., & Kojima, H. (1998). Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry. Journal of Fermentation and Bioengineering, 86(5), 521–522.
Hui, Y., Chao, L., Yang, D., Zhang, H., & Xi, Z. (2010). Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape and composition. Journal of Applied Toxicology, 29(1), 69–78.
Tao, B., Zhao, W., Lin, C., Yuan, Z., & Cai, K. (2020). Surface modification of titanium implants by ZIF-8@Levo/LBL coating for inhibition of bacterial-associated infection and enhancement of in vivo osseointegration. Chemical Engineering Journal, 390, 124621.
Dwyer, D. J., Kohanski, M. A., & Collins, J. J. (2009). Role of reactive oxygen species in antibiotic action and resistance. Current Opinion in Microbiology, 12(5), 482–489.
Imlay, J. A. (2013). The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nature Reviews Microbiology, 11, 443–454.
Xiu, Z. M., Zhang, Q. B., Puppala, H. L., Colvin, V. L., & Alvarez, P. J. J. (2012). Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Letters, 12(8), 4271–4275.
Jia, Z., et al. (2016). Bioinspired anchoring AgNPs onto micro-nanoporous TiO2 orthopedic coatings: Trap-killing of bacteria, surface-regulated osteoblast functions and host responses. Biomaterials, 75, 203–222.
Morones-Ramirez, J. R., Winkler, J. A., Spina, C. S., & Collins, J. J. (2013). Silver enhances antibiotic activity against gram-negative bacteria. Science Translational Medicine, 5(190), 190ra81.
Wang, X. H., et al. (2019). Osteogenic and antiseptic nanocoating by in situ chitosan regulated electrochemical deposition for promoting osseointegration. Materials Science & Engineering, C: Materials for Biological Applications, 102, 415–426.
Song, J. W., et al. (2020). Multifunctional antimicrobial biometallohydrogels based on amino acid coordinated self-assembly. Small (Weinheim an der Bergstrasse, Germany), 16(8), 1907309.
Mijnendonckx, K., Leys, N., Mahillon, J., Silver, S., & Houdt, R. V. (2013). Antimicrobial silver: Uses, toxicity and potential for resistance. BioMetals, 26(4), 609–621.
Li, W. R., Xie, X. B., Shi, Q. S., Zeng, H. Y., Ou-Yang, Y. S., & Chen, Y. B. (2010). Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Applied Microbiology and Biotechnology, 85(4), 1115–1122.
Kellici, S., et al. (2016). Calixarene assisted rapid synthesis of silver-graphene nanocomposites with enhanced antibacterial activity. ACS Applied Materials & Interfaces, 8(29), 19038–19046.
Wang, H. Y., Zhou, Y. F., Jiang, X. X., Sun, B., Zhu, Y., Wang, H., Su, Y. Y., & He, Y. (2015). Simultaneous capture, detection, and inactivation of bacteria as enabled by a surface-enhanced raman scattering multifunctional chip. Angewandte Chemie (International ed. in English), 54(17), 5132–5136.
Wahab, R., et al. (2014). ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids and Surfaces. B, Biointerfaces, 117(7), 267–276.
Jung, M., Kim, S., & Ju, S. (2011). Enhancement of green emission from Sn-doped ZnO nanowires. Optical Materials, 33(3), 280–283.
Jan, T., Iqbal, J., Ismail, M., Zakaullah, M., Naqvi, S. H., & Badshah, N. (2013). Sn doping induced enhancement in the activity of ZnO nanostructures against antibiotic resistant S. aureus bacteria. International Journal of Nanomedicine, 8, 3679–3687.
Sayilkan, F., Asiltürk, M., Kiraz, N., Burunkaya, E., Arpaç, E., & Sayilkan, E. (2009). Photocatalytic antibacterial performance of Sn(4+)-doped TiO(2) thin films on glass substrate. Journal of Hazardous Materials, 162(2–3), 1309–1316.
Jennings, M. C., Minbiole, K. P., & Wuest, W. M. (2015). Quaternary ammonium compounds: An antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS Infect. Dis., 1(7), 288–303.
Manaargadoo-Catin, M., Ali-Cherif, A., Pougnas, J. L., & Perrin, C. (2015). Hemolysis by surfactants—A review. Advances in Colloid and Interface Science, 228, 1–16.
Mitchell, M. A., Iannetta, A. A., Jennings, M. C., Fletcher, M. H., Wuest, W. M., & Minbiole, K. P. C. (2015). Scaffold-hopping of multicationic amphiphiles yields three new classes of antimicrobials. ChemBioChem, 16(16), 2299–2303.
Liu, F., He, D., Yu, Y., Cheng, L., & Zhang, S. (2019). Quaternary ammonium salt-based cross-linked micelles to combat biofilm. Bioconjugate Chemistry, 30(3), 541–546.
He, D., Yu, Y., Liu, F., Yao, Y., & Zhang, S. (2019). Quaternary ammonium salt-based cross-linked micelle templated synthesis of highly active silver nanocomposite for synergistic anti-biofilm application. Chemical Engineering Journal, 382, 122976.
Raafat, D., Bargen, K. V., Haas, A., & Sahl, H. G. (2008). Insights into the mode of action of chitosan as an antibacterial compound. Applied and Environment Microbiology, 74(12), 3764–3773.
Saravanan, S., Nethala, S., Pattnaik, S., Tripathi, A., Moorthi, A., & Selvamurugan, N. (2011). Preparation, characterization and antimicrobial activity of a bio-composite scaffold containing chitosan/nano-hydroxyapatite/nano-silver for bone tissue engineering. International Journal of Biological Macromolecules, 49(2), 188–193.
Hamed, I., Özogul, F., & Regenstein, J. M. (2016). Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends in Food Science & Technology, 48, 40–50.
Tarek, A., & Bader, A. (2016). Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Design, Development and Therapy, 10, 483–507.
Shariatinia, Z. (2018). Pharmaceutical applications of chitosan. Advances in Colloid and Interface Science, 263, 131–194.
Jiang, F., Deng, Y., Yeh, C. K., & Sun, Y. (2014). Quaternized chitosans bind onto preexisting biofilms and eradicate pre-attached microorganisms. J. Mater. Chem. B., 2(48), 8518–8527.
Paz, L. E. C., Resin, A., Howard, K. A., Sutherland, D. S., & Wejse, P. L. (2011). Antimicrobial effect of chitosan nanoparticles on Streptococcus mutans biofilms. Applied and Environment Microbiology, 77(11), 3892–3895.
Ing, L. Y., Zin, N. M., Sarwar, A., & Katas, H. (2012). Antifungal activity of chitosan nanoparticles and correlation with their physical properties. International Journal of Biomaterials, 2012, 632698.
Darouiche, R. O. (2004). Treatment of infections associated with surgical implants. The New England Journal of Medicine, 172(5), 2102.
Al-Radha, A. S. D., Dymock, D., Younes, C., & O’Sullivan, D. (2012). Surface properties of titanium and zirconia dental implant materials and their effect on bacterial adhesion. Journal of Dentistry, 40(2), 146–153.
Lv, H., Chen, Z., Yang, X., Cen, L., Zhang, X., & Gao, P. (2014). Layer-by-layer self-assembly of minocycline-loaded chitosan/alginate multilayer on titanium substrates to inhibit biofilm formation. Journal of Dentistry, 42(11), 1464–1472.
Subhaswaraj, P., Barik, S., Macha, C., Chiranjeevi, P. V., & Siddhardha, B. (2018). Anti quorum sensing and anti biofilm efficacy of cinnamaldehyde encapsulated chitosan nanoparticles against Pseudomonas aeruginosa PAO1. Journal of Food Science and Technology, 97, 752–759.
Fu, J., Ji, J., Yuan, W., & Shen, J. (2005). Construction of anti-adhesive and antibacterial multilayer films via layer-by-layer assembly of heparin and chitosan. Biomaterials, 26(33), 6684–6692.
Masood, N., et al. (2019). Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. International Journal of Pharmaceutics, 559, 23–36.
Lodhi, G., et al. (2014). Chitooligosaccharide and its derivatives: preparation and biological applications. BioMed Research International, 2014(1), 654913.
Negm, N. A., Hefni, H. H. H., Abd-Elaal, A. A. A., Badr, E. A., & Kana, M. T. H. (2020). Advancement on modification of chitosan biopolymer and its potential applications. International Journal of Biological Macromolecules, 152, 681–702.
Khan, F., Lee, J. W., Pham, D. T. N., & Kim, Y. M. (2019). Chitooligosaccharides as antibacterial, antibiofilm, antihemolytic and anti-virulence agent against Staphylococcus aureus. Current Pharmaceutical Biotechnology, 20(14), 1223–1233.
He, X., Hwang, H. M., Aker, W. G., Wang, P., Lin, Y., Jiang, X., & He, X. (2014). Synergistic combination of marine oligosaccharides and azithromycin against Pseudomonas aeruginosa. Microbiological Research, 169(9–10), 759–767.
Barraud, N., Hassett, D. J., Hwang, S. H., Rice, S. A., Kjelleberg, S., & Webb, J. S. (2006). Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. Journal of Bacteriology, 188(21), 7344–7353.
Barraud, N., Schleheck, D., Klebensberger, J., Webb, J. S., Hassett, D. J., Rice, S. A., & Kjelleberg, S. (2009). Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal. Journal of Bacteriology, 191(23), 7333–7342.
Rinaldo, S., Giardina, G., Mantoni, F., Paone, A., & Cutruzzolà, F. (2018). Beyond nitrogen metabolism: nitric oxide, cyclic-di-GMP and bacterial biofilms. FEMS Microbiology Letters, 365(6), fny029.
Reighard, K. P., Hill, D. B., Dixon, G. A., Worley, B. V., & Schoenfisch, M. H. (2015). Disruption and eradication of P. aeruginosa biofilms using nitric oxide-releasing chitosan oligosaccharides. Biofouling, 31(9–10), 775–787.
Peng, Z., Wang, L., Du, L., Guo, S., Wang, X., & Tang, T. (2010). Adjustment of the antibacterial activity and biocompatibility of hydroxypropyltrimethyl ammonium chloride chitosan by varying the degree of substitution of quaternary ammonium. Carbohydrate Polymers, 81(2), 275–283.
Zhao, X., Li, P., Guo, B., & Ma, P. X. (2015). Antibacterial and conductive injectable hydrogels based on quaternized chitosan-graft-polyaniline/oxidized dextran for tissue engineering. Acta Biomaterialia, 26, 236–248.
He, J., Liang, Y., Shi, M., & Guo, B. (2019). Anti-oxidant electroactive and antibacterial nanofibrous wound dressings based on poly(ε-caprolactone)/quaternized chitosan-graft-polyaniline for full-thickness skin wound healing. Chemical Engineering Journal, 385, 123464.
Zheng, Z., Bian, S., Li, Z., Zhang, Z., & Zhao, X. (2020). Catechol modified quaternized chitosan enhanced wet adhesive and antibacterial properties of injectable thermo-sensitive hydrogel for wound healing. Carbohydrate Polymers, 249, 116826.
Khan, F., Pham, D. T. N., Oloketuyi, S. F., Manivasagan, P., & Kim, Y. M. (2019). Chitosan and their derivatives: antibiofilm drugs against pathogenic bacteria. Colloids and Surfaces. B, Biointerfaces, 185, 110627.
Goy, R. C., Briito, D., & Assis, O. B. G. (2009). A review of the antimicrobial activity of chitosan. Polímeros, 19(3), 241–247.
Ageitos, J. M., Sánchez-Pérez, A., Calo-Mata, P., & Villa, T. G. (2017). Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochemical Pharmacology, 133, 117–138.
Torres, M. D. T., Sothiselvam, S., Lu, T. K., & Fuente-Nunez, C. (2019). Peptide design principles for antimicrobial applications. Journal of Molecular Biology, 431(18), 3547–3567.
Mookherjee, N., Anderson, M. A., Haagsman, H. P., & Davidson, D. J. (2020). Antimicrobial host defence peptides: Functions and clinical potential. Nature Reviews. Drug Discovery, 19(5), 311–332.
Qi, F., et al. (2019). Practical preparation of infection-resistant biomedical surfaces from antimicrobial β-peptide polymers. ACS Applied Materials & Interfaces, 11(21), 18907–18913.
Zhang, Q., et al. (2019). Host defense peptide mimicking poly-β-peptides with fast, potent and broad spectrum antibacterial activities. Biomater. Sci., 7(5), 2144–2151.
Zhou, M., Qian, Y., Xie, J., Zhang, W., & Liu, R. (2020). Poly(2-oxazoline)-based functional peptide mimics: Eradicating MRSA infections and persisters while alleviating antimicrobial resistance. Angewandte Chemie (International ed. in English), 59(16), 6412–6419.
Dai, C., Zhou, M., Jiang, W., Xiao, X., & Liu, R. (2020). Breaking or following the membrane-targeting mechanism: Exploring the antibacterial mechanism of host defense peptide mimicking poly(2-oxazoline)s. Journal of Materials Science and Technology, 59, 220–226.
Cascales, E., Buchanan, S. K., Duché, D., Kleanthous, C., Lloubès, R., Postle, K., Riley, M., Slatin, S., & Cavard, D. (2007). Colicin Biology, 71(1), 158–229.
Meade, E., Slattery, M. A., & Garvey, M. (2020). Bacteriocins, Potent antimicrobial peptides and the fight against multi drug resistant species: Resistance is futile? Antibiotics, 9(1), 32.
Shin, J. M., Gwak, J. W., Kamarajan, P., Fenno, J. C., Rickard, A. H., & Kapila, Y. L. (2016). Biomedical applications of nisin. Journal of Applied Microbiology, 120(6), 1449–1465.
Costerton, J. W. (1999). Bacterial Biofilms: A common cause of persistent infections. Science, 284(5418), 1318–1322.
Yu, Q., Wu, Z., & Chen, H. (2015). Dual-function antibacterial surfaces for biomedical applications. Acta Biomaterialia, 16(1), 1–13.
Schild, H. G. (1992). Poly(N-isopropylacrylamide): Experiment, theory and application. Progress in Polymer Science, 17(2), 163–249.
Ista, L. K., Mendez, S., & Lopez, G. P. (2010). Attachment and detachment of bacteria on surfaces with tunable and switchable wettability. Biofouling, 26(1–2), 111–118.
Cunliffe, D., Smart, C. A., Tsibouklis, J., Young, S., Alexander, C., & Vulfson, E. N. (2000). Bacterial adsorption to thermoresponsive polymer surfaces. Biotechnology Letters, 22(2), 141–145.
Cunliffe, D., Carolina, D., Peters, V., Smith, J. R., & Alexander, C. (2003). Thermoresponsive surface-grafted poly(N-isopropylacrylamide) copolymers: Effect of phase transitions on protein and bacterial attachment. Langmuir, 19(7), 2888–2899.
Yu, Q., Cho, J., Shivapooja, P., Ista, L. K., & López, G. P. (2013). Nanopatterned smart polymer surfaces for controlled attachment, killing, and release of bacteria. ACS Applied Materials & Interfaces, 5(19), 9295–9304.
Luo, M., Bernards, M. T., Gang, C., Yu, Q., & Jiang, S. (2010). pH responsive properties of non-fouling mixed-charge polymer brushes based on quaternary amine and carboxylic acid monomers. Biomaterials, 31(10), 2919–2925.
Makhathini, S. S., Omolo, C. A., Gannimani, R., Mocktar, C., & Govender, T. (2020). pH-Responsive micelles from an oleic acid tail and propionic acid heads dendritic amphiphile for the delivery of antibiotics. Journal of Pharmaceutical Sciences, 109(8), 2594–2606.
Yang, N., Zhu, M., Xu, G., Liu, N., & Yu, C. (2020). A near-infrared light-responsive multifunctional nanocomposite hydrogel for efficient and synergistic antibacterial wound therapy and healing promotion. Journal of Materials Chemistry B, 8(17), 3908–3917.
Zhao, X., Liang, Y., Huang, Y., He, J., Han, Y., & Guo, B. (2020). Physical double-network hydrogel adhesives with rapid shape adaptability, fast self-healing, antioxidant and NIR/pH stimulus-responsiveness for multidrug-resistant bacterial infection and removable wound dressing. Advanced Functional Materials, 30(17), 1910748.
Banerjee, R., Phan, A., Wang, B., Knobler, C., Furukawa, H., O’Keeffe, M., & Yaghi, O. M. (2008). High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science, 319(5865), 939–943.
Rocca, J. D., Liu, D., & Lin, W. (2011). Nanoscale metal-organic frameworks for biomedical imaging and drug delivery. Accounts of Chemical Research, 44(10), 957–968.
Imaz, I., Hernando, J., Ruiz-Molina, D., & Maspoch, D. (2010). Metal-organic spheres as functional systems for guest encapsulation. Angewandte Chemie (International ed. in English), 48(13), 2325–2329.
Liang, K., Coghlan, C. J., Bell, S. G., Doonan, C., & Falcaro, P. (2016). Enzyme encapsulation in zeolitic imidazolate frameworks: A comparison between controlled co-precipitation and biomimetic mineralisation. Chemical Communications, 52(3), 473–476.
He, M., Yao, J., Liu, Q., Wang, K., Chen, F., & Wang, H. (2014). Facile synthesis of zeolitic imidazolate framework-8 from a concentrated aqueous solution. Microporous and Mesoporous Materials, 184, 55–60.
Song, Z., Wu, Y., Cao, Q., Wang, H., & Xiang, H. (2018). pH-responsive, light-triggered on-demand antibiotic release from functional metal-organic framework for bacterial infection combination therapy. Advanced Functional Materials, 28(23), 1800011.
Tkhilaishvili, T., Winkler, T., Müller, M., Perka, C., & Trampuz, A. (2019). Bacteriophages as adjuvant to antibiotics for the treatment of periprosthetic joint infection caused by multidrug-resistant Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy, 64(1), e00924-e1019.
Gu, J., Liu, X., Li, Y., Han, W., & Lei, L. (2012). A method for generation phage cocktail with great therapeutic potential. PLoS ONE, 7(3), e31698.
Deresinski, S. (2009). Bacteriophage therapy: Exploiting smaller fleas. Clinical Infectious Diseases, 48(8), 1096–1101.
Fish, R., Kutter, E., Wheat, G., Blasdel, B., & Kuhl, S. (2018). Compassionate use of bacteriophage therapy for foot ulcer treatment as an effective step for moving toward clinical trials. Methods in Molecular Biology, 1693, 159–170.
Kakasis, A., & Panitsa, G. (2019). Bacteriophage therapy as an alternative treatment for human infections. A comprehensive review. International Journal of Antimicrobial Agents, 53(1), 16–21.
Lin, D. M., Koskella, B., & Lin, H. C. (2017). Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Ther., 8(3), 162–173.
Erez, Z., Steinberger-Levy, I., Shamir, M., Doron, S., Stokar-Avihail, A., Peleg, Y., Melamed, S., Leavitt, A., Savidor, A., Albeck, S., & Amitai, G. (2017). Communication between viruses guides lysis-lysogeny decisions. Nature, 541(7638), 488–488.
Clark, J. R. (2015). Bacteriophage therapy: history and future prospects. Future Virology, 10(4), 449–461.
Caflisch, K. M., & Patel, R. (2019). Implications of bacteriophage- and bacteriophage component-based therapies for the clinical microbiology laboratory. Journal of Clinical Microbiology, 57(8), e00229-19.
Mirzaei, M. K., & Nilsson, A. S. (2015). Isolation of phages for phage therapy: a comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS ONE, 10(5), e0127606.
Chaudhry, W. N., Concepción-Acevedo, J., Park, T., Andleeb, S., & Levin, B. R. (2017). Synergy and order effects of antibiotics and phages in killing Pseudomonas aeruginosa biofilms. PLoS ONE, 12(1), e0168615.
Tanji, Y., Shimada, T., Yoichi, M., Miyanaga, K., Hori, K., & Unno, H. (2004). Toward rational control of Escherichia coli O157:H7 by a phage cocktail. Applied Microbiology and Biotechnology, 64(2), 270–274.
Jault, P., Leclerc, T., Jennes, S., Pirnay, J. P., Que, Y. A., Resch, G., Rousseau, A. F., Ravat, F., Carsin, H., & Floch, R. L. (2018). Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. The Lancet Infectious Diseases, 19(1), 35–45.
Wang, Y., Beekman, J., Hew, J., Jackson, S., et al. (2017). Burn injury: Challenges and advances in burn wound healing, infection, pain and scarrin. Advanced Drug Delivery Reviews, 123(1), 3–17.
Schmelcher, M., Donovan, D. M., & Loessner, M. J. (2012). Bacteriophage endolysins as novel antimicrobials. Future Microbiology, 7(10), 1147–1171.
Das, T., Krom, B. P., Mei, H., Busscher, H. J., & Sharma, P. K. (2011). DNA-mediated bacterial aggregation is dictated by acid–base interactions. Soft Matter, 7(6), 2927–2935.
Swartjes, J. J., Das, T., Sharifi, S., & Subbiahdoss, G. (2013). A functional DNase I coating to prevent adhesion of bacteria and the formation of biofilm. Advanced Functional Materials, 23(22), 2843–2849.
Banerjee, I., Pangule, R. C., & Kane, R. S. (2011). Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Advanced Materials, 23(6), 690–718.
Liu, T., Yan, S., Zhou, R., Zhang, X., & Luan, S. (2020). Self-adaptive antibacterial coating for universal polymeric substrates based on micrometer-scale hierarchical polymer brush system. ACS Applied Materials & Interfaces, 12(38), 42576–42585.
Gu, P., Fan, N., Wang, Y., Wang, J., & Zhong, Q. (2019). Linear control of moisture permeability and anti-adhesion of bacteria in a broad temperature region realized by cross-linking thermoresponsive microgels onto cotton fabrics. ACS Applied Materials & Interfaces, 11(33), 30269–30277.
Cirioni, O., Giacometti, A., Ghiselli, R., Acqua, G. D., Orlando, F., Mocchegiani, F., Silvestri, C., Licci, A., Saba, V., & Scalise, G. (2006). RNAIII-inhibiting peptide significantly reduces bacterial load and enhances the effect of antibiotics in the treatment of central venous catheter-associated Staphylococcus aureus infections. Journal of Infectious Diseases, 193(2), 180–186.
Naomi, B., Andrea, G., Oscar, C., Ghiselli, Y., & Mocchegiani, R. (2003). Use of the quorum-sensing inhibitor RNAIII-inhibiting peptide to prevent biofilm formation in vivo by drug-resistant Staphylococcus epidermidis. Journal of Infectious Diseases, 187(4), 625–630.
Stones, D. H., & Krachler, A. M. (2016). Against the tide: The role of bacterial adhesion in host colonization. Biochemical Society Transactions, 44(6), 1571–1580.
He, J., She, M., Liang, Y., & Guo, B. (2020). Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chemical Engineering Journal, 394, 124888.
Drescher, K., Shen, Y., Bassler, B. L., & Stone, H. A. (2013). Biofilm streamers cause catastrophic disruption of flow with consequences for environmental and medical systems. Proceedings of the National Academy of Sciences, 110(11), 4345–4350.
Brogden, K. A., Guthmiller, J. M., & Taylor, C. E. (2005). Human polymicrobial infections. Lancet, 365(9455), 253–255.
Acknowledgements
This work is financially supported by National Natural Science Foundation of China (51903176), Clinical Research Incubation Project (No. 2019HXFH006), and Special Fund for Nursing Discipline Development (No. HXHL19003) of Discipline Excellence Development 135 Project of West China Hospital of Sichuan University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, Q., Hu, X. & Wang, Y. Alternatives to Conventional Antibiotic Therapy: Potential Therapeutic Strategies of Combating Antimicrobial-Resistance and Biofilm-Related Infections. Mol Biotechnol 63, 1103–1124 (2021). https://doi.org/10.1007/s12033-021-00371-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-021-00371-2