Abstract
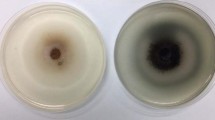
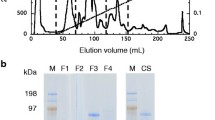
Enzymatic hydrolysis of cellulosic biomass has caught much attention because of modest reaction conditions and environment friendly conditions. To reduce the cost and to achieve good quantity of cellulases, a heterologous expression system is highly favored. In this study, cellulose-degrading enzymes, GH3 family β-glucosidase (BGL), GH7 family-related cellobiohydrolases (CBHs), and endoglucanase (EG) from a newly isolated Aspergillus niger BE-2 are highly expressed in Pichia pastoris GS115. The strain produced EG, CBHs, and BGL enzymatic concentration of 0.56, 0.11, and 22 IU/mL, respectively. Mode of actions of the recombinant enzymes for substrate specificity and end product analysis are verified and found specific for cellulose degradation. Bamboo biomass saccharification with A. niger cellulase released a high level of fermentable sugars. Hydrolysis parameters are optimized to obtain reducing sugars level of 3.18 g/L. To obtain reducing sugars from a cellulosic biomass, A. niger could be a good candidate for enzymes resource of cellulase to produce reducing sugars from a cellulosic biomass. This study also facilitates the development of highly efficient enzyme cocktails for the bioconversion of lignocellulosic biomass into monosaccharides and oligosaccharides.






Similar content being viewed by others
References
Lynd, L. R., Weimer, P. J., van Zyl, W. H., & Pretorius, I. S. (2002). Microbial cellulose utilization: Fundamentals and biotechnology. Microbiology and Molecular Biology Reviews, 66, 506–577.
Esterbauer, H., Steiner, W., Labudova, I., Hermann, A., & Hayn, M. (1991). Production of Trichoderma cellulase in laboratory and pilot plant. Bioresource Technology, 36, 67–76.
Bagga, P. S., Sandhu, D. K., & Sharma, S. (1990). Purification and characterization of cellulolytic enzymes produced by Aspergillus nidulans. Journal of Applied Bacteriology, 68, 61–68.
Vidmar, S., Turk, V., & Kregar, I. (1984). Cellulolytic complex of Aspergillus niger under conditions for citric acid production. Isolation and characterization of two b-(1 → 4)-glucan hydrolases. Applied Microbiol Biotechnology, 20, 326–330.
Gadgil, N. J., Daginawala, H. F., Chakarabarti, T., & Khanna, P. (1995). Enhanced cellulase production by a mutant of Trichoderma reesei. Enzyme Microbial Technology, 17, 942–946.
Singhania, R. R., Patel, A. K., Sukumaran, R. K., Larroche, C., & Pandey, A. (2012). Role and significance of β-glucosidases in the hydrolysis of cellulose for bioethanol production. Bioresource Technology, 127, 500–507.
Mooney, C. A., Mansfield, S. D., Touhy, M. G., & Saddler, J. N. (1998). The effect of initial pore volume and lignin content on the enzymatic hydrolysis of softwoods. Bioresource Technology, 64, 113–119.
Sun, Y., & Cheng, J. (2002). Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresource Technology, 83, 1–11.
Wilkins, M. R., Widmer, W. W., Grohmann, K., & Cameron, R. G. (2007). Hydrolysis of grapefruit peel waste with cellulase and pectinase enzymes. Bioresource Technology, 98, 1596–1601.
Persson, I., Tjerneld, F., & Hahn-Hagerdal, B. B. (1991). Fungal cellulolytic enzyme production: A review. Process Biochemistry, 26, 65–74.
Holtzapple, M., Cognata, M., Shu, Y., & Hendrickson, C. (1990). Inhibition of Trichoderma reesei cellulase by sugars and solvents. Biotechnology Bioengineering, 36, 275–287.
Wright, J. D., Power, A. J., & Douglas, L. J. (1986). Design and parameter evaluation of an enzymatic hydrolysis process (separation hydrolysis and fermentation). Biotechnology and Bioengineering, 17, 285–302.
Ferreira, S., Duarte, A. P., Ribeiro, M. H. L., Queiroz, J. A., & Domingues, F. C. (2009). Response surface optimization of enzymatic hydrolysis of Cistus ladanifer and Cytisusstriatus for bioethanol production. Biochemical Engineering, 45, 192–200.
Cardona, C. A., & Sanchez, C. J. (2007). Fuel ethanol production: Process design trends and integration opportunities. Bioresource Technology, 98, 2415–2457.
He, M. X., Wang, J. L., Qin, H., Shui, Z. X., Zhu, Q. L., Wu, B., et al. (2014). Bamboo: A new source of carbohydrate for biorefinery. Carbohydrate Polymers, 111, 645–654.
Amada, S., Munekata, T., Nagase, Y., Ichikawa, Y., Kirigai, A., & Zhifei, Y. (1996). Themechanical structures of bamboos in viewpoint of functionally gradient and composite materials. Journal of Composite Materials, 30, 800–819. doi:10.1177/002199839603000703.
Choy, K. K. H., Barford, J. P., & McKay, G. (2005). Production of activated carbon from bamboo scaffolding waste process design, evaluation and sensitivity analysis. Chemical Engineering, 109, 147–165. doi:10.1016/j.cej.2005.02.030.
Yang, H., Lirong, T., Qilin, L., Siqun, W., Xuerong, C., & Biao, H. (2014). Preparation of cellulose nanocrystals and carboxylated cellulose nanocrystals from borer powder of bamboo. Cellulose, 21, 1611–1618. doi:10.1007/s10570-014-0236-0.
Balatinecz, J. J., & Kretschmann, D. E. (2001). Properties and utilization of poplar wood (pp. 277–291). Ottawa, ON: NRC Research Press, National Research Council of Canada.
Juturu, V., & Wu, J. C. (2012). Microbial xylanases: Engineering, production and industrial applications. Biotechnology Advances, 30, 1219–1227.
Laemmli, U. K. (1970). Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of micorgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Somogyi, M. (1951). Notes on sugar determination. Journal of Biological Chemistry, 195, 19–23.
Riou, C., Salmon, J. M., Vallier, M. J., Gunata, Z., & Barre, P. (1998). Purification, characterization and substrate specificity of a novel highly glucose-tolerant β-glucosidase from Aspergillus oryzae. Applied Environmental Microbiology, 64, 3607–3614.
Miller, G. L. (1959). Use of dinitrosalycilic acid reagent for determination of reducing sugars. Analytical Chemistry, 31, 426–430.
Li, H., Long, C., Zhou, J., Liu, J., Wu, X., & Long, M. (2013). Rapid analysis of mono-saccharides and oligo-saccharides in hydrolysates of lignocellulosic biomass by HPLC. Biotechnology Letter, 35, 1405–1409.
Yamashita, Y., Shono, M., Sasaki, C., & Nakamura, Y. (2010). Alkaline peroxide pretreatment for efficient enzymatic saccharification of bamboo. Carbohydrate Polymers, 79, 914–920.
Goering, H. K., & Van Soest, P. J. (1970). Forage fibre analysis. Agriculture handbook (pp. 387–598). Washington, DC: Agricultural Research Services, United States Department of Agriculture.
Jiwei, Z., Yaohua, Z., Xuena, Z., & Tianhong, W. (2010). Development of the cellulolytic fungus Trichoderma reesei strain with enhanced β-glucosidase and filter paper activity using strong artificial cellobiohydrolase-1 promoter. Bioresource Technology, 101, 9815–9818. doi:10.1016/j.biortech.2010.07.078.
Marimuthu, J., Ngoc, P. N., Hee, J. M., Sang, K., & Jung, K. L. (2010). Conversion of woody biomass into fermentable sugars by cellulase from Agaricus arvensis. Bioresource Technology, 101, 8742–8749.
Guoqing, L., Changsheng, C., Song, F., & Linguo, Z. (2012). Cloning of a cellobiohydrolase gene (cbh1) from Aspergillus niger and heterogenous expression in Pichia pastoris. Advanced Materials Research, 353, 2443–2447.
Teeri, T., Lehtovaara, P., Kauppinen, S., Salovuori, I., & Knowles, J. (1987). Homologous domains in Trichoderma reesei cellulolytic enzymes: Gene sequence and expression of cellobiohydrolase II. Gene, 51, 43–52.
Koch, A., Weigel, C. T. O., & Schulz, G. (1993). Cloning sequencing and heterologous expression of a cellulase 2 encoding cDNA from P. janthinellum. Gene, 124, 57–65.
Covert, S. F., Vanden, W. A., & Cullen, D. (1992). Structure, organization, and transcription of a cellobiohydrolase gene cluster from Phanerochaete chrysosporium. Applied Environmental Microbiology, 58, 2168–2175.
Takada, G., Kawaguchi, T., Sumitani, J., & Arai, M. (1998). Expression of Aspergillus aculeatus No. F-50 cellobiohydrolase I (cbhI) and beta-glucosidase 1 (bgl1) genes by Saccharomyces cerevisiae. Bioscience, Biotechnology, and Biochemistry, 3, 1615–1618.
Hamada, N., Fuse, N., Shimosaka, M., Kodaira, R., Amano, Y., Kanda, T., & Okazaki, M. (1999). Cloning and characterization of a new exo-cellulase gene, cel3, in Irpex lacteus. FEMS Microbiology Letters, 172, 231–237.
Hu, J., Arantes, V., Pribowo, A., & Saddler, J. N. (2013). The synergistic action of accessory enzymes enhances the hydrolytic potential of a ‘cellulase mixture’ but is highly substrate specific. Biotechnology Biofuels, 6, 112.
Zhang, J., Siika-aho, M., Puranen, T., Tang, M., Tenkanen, M., & Viikari, L. (2011). Thermostable recombinant xylanases from Nonomuraea flexuosa and Thermoascus aurantiacus show distinct properties in the hydrolysis of xylans and pretreated wheat straw. Biotechnology Biofuels, 4, 12.
Sewalt, V. J. H., Beauchemin, K. A., Rode, L. M., Acharya, S., & Baron, V. S. (1997). Lignin impact on fiber degradation. IV. Enzymatic saccharification and in vitro digestibility of alfalfa and grasses following selective solvent delignification. Bioresource Technology, 61, 199–206.
Kim, T. H., & Lee, Y. Y. (2005). Pretreatment and fractionation of corn stover by ammonia recycle percolation process. Bioresource Technology, 96, 2007–2013.
Xu, Z., Wang, Q. H., Jiang, Z. H., Yang, X. X., & Ji, Y. Z. (2007). Enzymatic hydrolysis of pretreated soybean straw. Biomass and Bioenergy, 31, 162–167.
Zhang, Q., & Cai, W. (2008). Enzymatic hydrolysis of alkali-pretreated rice straw by Trichoderma reesei ZM4-F3. Biomass and Bioenergy, 32, 1130–1135.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31170067, 21303142), the research fund of Fujian Provincial Natural Science Foundation (Grant No: 2012J05029), and the National Basic Research Program of China (973 Program, Grant No: 2010CB732201).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ali, N., Ting, Z., Li, H. et al. Heterogeneous Expression and Functional Characterization of Cellulose-Degrading Enzymes from Aspergillus niger for Enzymatic Hydrolysis of Alkali Pretreated Bamboo Biomass. Mol Biotechnol 57, 859–867 (2015). https://doi.org/10.1007/s12033-015-9878-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-015-9878-x