Abstract
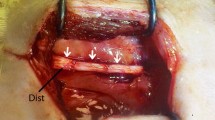
Adipose-derived stem cells (ADSCs) are a desirable stem cell source in neurodegenerative diseases treatment due to their ability to differentiate into different cell lineages. In this study, we transplanted human ADSCs (hADSCs) into a lysophosphatidylcholine (lysolecithin) model of multiple sclerosis (MS) and determined the efficiency of these cells in remyelination process. Forty adult rats were randomly divided into control, lysolecithin, vehicle, and transplantation groups, and focal demyelination was induced by lysolecithin injection into spinal cord. To assess motor performance, all rats were examined weekly with a standard EAE scoring scale. Four weeks after cell transplantation, to assess the extent of demyelination and remyelination, Luxol Fast Blue staining was used. In addition, immunohistochemistry technique was used for assessment of the presence of oligodendrocyte phenotype cells in damaged spinal cord. Our results indicated that hADSCs had ability to differentiate into oligodendrocyte phenotype cells and improved remyelination process. Moreover, the evaluation of rat motor functions showed that animals which were treated with hADSC compared to other groups had significant improvement (P < 0.001). Our finding showed that hADSCs transplantation for cell-based therapies may play a proper cell source in the treatment of neurodegenerative diseases such as MS.





Similar content being viewed by others
References
Compston, A., & Coles, A. (2008). Multiple sclerosis. Lancet, 372(9648), 1502–1517.
Khan, F., Turner-Stokes, L., Ng, L., & Kilpatrick, T. (2008). Multidisciplinary rehabilitation for adults with multiple sclerosis. Journal of Neurology, Neurosurgery and Psychiatry, 79(2), 114.
Holloman, J. P., Ho, C. C., Hukki, A., Huntley, J. L., & Gallicano, G. I. (2013). The development of hematopoietic and mesenchymal stem cell transplantation as an effective treatment for multiple sclerosis. American Journal of Stem Cells, 2(2), 95–107.
Hatch, M. N., Schaumburg, C. S., Lane, T. E., & Keirstead, H. S. (2009). Endogenous remyelination is induced by transplant rejection in a viral model of multiple sclerosis. Journal of Neuroimmunology, 212(1–2), 74–81.
Barhum, Y., Gai-Castro, S., Bahat-Stromza, M., Barzilay, R., Melamed, E., & Offen, D. (2010). Intracerebroventricular transplantation of human mesenchymal stem cells induced to secrete neurotrophic factors attenuates clinical symptoms in a mouse model of multiple sclerosis. The Journal of Molecular Neuroscience, 41(1), 129–137.
Kulbatski, I., Mothe, A. J., Keating, A., Hakamata, Y., Kobayashi, E., & Tator, C. H. (2007). Oligodendrocytes and radial glia derived from adult rat spinal cord progenitors: Morphological and immunocytochemical characterization. Journal of Histochemistry and Cytochemistry, 55(3), 209–222.
Fraser, J. K., Wulur, I., Alfonso, Z., et al. (2006). Fat tissue: An underappreciated source of stem cells for biotechnology. Trends in Biotechnology, 24(4), 150–154.
Parker, A. M., & Katz, A. J. (2006). Adipose-derived stem cells for the regeneration of damaged tissues. Expert Opinion on Biological Therapy, 6(6), 567–578.
Kalbermatten, D. F., Schaakxs, D., Kingham, P. J., & Wiberg, M. (2011). Neurotrophic activity of human adipose stem cells isolated from deep and superficial layers of abdominal fat. Cell and Tissue Research, 344(2), 251–260.
Guilak, F., Lott, K. E., Awad, H. A., Cao, Q., Hicok, K. C., Fermor, B., et al. (2006). Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. Journal of Cellular Physiology, 206(1), 229–237.
Razavi, S., Razavi, M. R., Zarkesh Esfahani, H., Kazemi, M., & Mostafavi, F. S. (2013). Comparing brain-derived neurotrophic factor and ciliary neurotrophic factor secretion of induced neurotrophic factor secreting cells from human adipose and bone marrow-derived stem cells. Development, Growth and Differentiation, 55(6), 648–655.
Razavi, S., Mardani, M., Kazemi, M., Esfandiari, E., Narimani, M., Esmaeili, A., et al. (2013). Effect of leukemia inhibitory factor on the myelinogenic ability of Schwann-like cells induced from human adipose-derived stem cells. Cellular and Molecular Neurobiology, 33(2), 283–289.
Orbay, H., Uysal, A. C., Hyakusoku, H., & Mizuno, H. (2012). Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. Journal of Plastic, Reconstructive and Aesthetic Surgery, 65(5), 657–664.
Degaonkar, M. N., Raghunathan, P., Jayasundar, R., & Jagannathan, N. R. (2005). Determination of relaxation characteristics during preacute stage of lysophosphatidyl choline-induced demyelinating lesion in rat brain: an animal model of multiple sclerosis. Magnetic Resonance Imaging, 23(1), 69–73.
Degaonkar, M. N., Jayasundar, R., & Jagannathan, N. R. (2002). Sequential diffusion-weighted magnetic resonance imaging study of lysophosphatidyl choline-induced experimental demyelinating lesion: an animal model of multiple sclerosis. Journal of Magnetic Resonance Imaging, 16(2), 153–159.
Ahmadi, N., Razavi, S., Kazemi, M., & Oryan, S. (2012). Stability of neural differentiation in human adipose derived stem cells by two induction protocols. Tissue and Cell, 44, 87–94.
Boido, M., Garbossa, D., & Vercelli, A. (2011). Early graft of neural precursors in spinal cord compression reduces glial cyst and improves function. Journal of Neurosurgery, 15(1), 97–106.
Kerschensteiner, M., Stadelmann, C., Buddeberg, B. S., Merkler, D., Bareyre, F. M., Anthony, D. C., et al. (2004). Targeting experimental autoimmune encephalomyelitis lesions to a predetermined axonal tract system allows for refined behavioral testing in an animal model of multiple sclerosis. American Journal of Pathology, 164(4), 1455–1469.
Mikaeili Agah, E., Parivar, K., Joghataei, MT. (2013). Therapeutic effect of transplanted human Wharton’s Jelly stem cell-derived oligodendrocyte progenitor cells (hWJ-MSC-derived OPCs) in an Animal model of multiple sclerosis. Molecular Neurobiology. doi:10.1007/s12035-013-8543-2.
Flachenecker, P., & Stuke, K. (2008). National MS registries. Journal of Neurology, 255(6), 102–108.
Hasan, K. M., Walimuni, I. S., Abid, H., Datta, S., Wolinsky, J. S., & Narayana, P. A. (2012). Human brain atlas-based multi-modal MRI analysis of volumetry, diffusometry, re-laxometry and lesion distribution in multiple sclero-sis patients and healthy adult controls: implications for understanding the pathogenesis of multiple scle-rosis and consolidation of quantitative MRI results in MS. The Journal of the Neurological Sciences, 313(2), 99–109.
Kidd, P. (2003). Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Alternative Medicine Review, 8(3), 223–246.
Roncarolo, M. G., Bacchetta, R., Bordignon, C., et al. (2001). Type 1 T regulatory cells. Immunological Reviews, 182, 68–79.
MohyeddinBonab, M., Mohajeri, M., Sahraian, M. A., Yazdanifar, M., Aghsaie, A., Farazmand, A., et al. (2013). Evaluation of cytokines in multiple sclerosis patients treated with mesenchymal stem cells. Archives of Medical Research, 44(4), 266–272.
Constantin, G., Marconi, S., Rossi, B., Angiari, S., Cal-deran, L., Anghileri, E., et al. (2009). Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells, 27(10), 2624–2635.
Wei, X., Zhao, L., Zhong, J., Gu, H., Feng, D., Johnstone, B. H., et al. (2009). Adipose stromal cells-secreted neuroprotective media against neuronal apoptosis. Neuroscience Letters, 462(1), 76–79.
Sen, A., Lea-Currie, Y. R., Sujkowska, D., Franklin, D. M., Wilkison, W. O., Halvorsen, Y. D., et al. (2001). Adipogenic potential of human adipose derived stromal cells from multiple donors is heterogeneous. Journal of Cellular Biochemistry, 81, 312–319.
Fuhrmann, T., Montzka, K., Hillen, L. M., Hodde, D., Dreier, A., Bozkurt, A., et al. (2010). Axon growth-promoting properties of human bone marrow mesenchymal stromal cells. Neuroscience Letters, 474(1), 37–41.
Decker, L., Desmarquet-Trin-Dinh, C., Taillebourg, E., Ghislain, J., Vallat, J. M., & Charnay, P. (2006). Peripheral myelin maintenance is a dynamic process requiring constant Krox20 expression. Journal of Neuroscience, 26(38), 9771–9779.
Jahn, O., Tenzer, S., & Werner, H. B. (2009). Myelin proteomics: molecular anatomy of an insulating sheath. Molecular Neurobiology, 40(1), 55–72.
Jaramillo-Merchán, J., Jones, J., Ivorra, J. L., Pastor, D., Viso-León, M. C., Armengól, J. A., et al. (2013). Mesenchymal stromal-cell transplants induce oligodendrocyte progenitor migration and remyelination in a chronic demyelination model. Cell Death and Disease, 4, e779.
Bai, L., Hecker, J., Kerstetter, A., & Miller, R. H. (2013). Myelin repair and functional recovery mediated by neural cell transplantation in a mouse model of multiplesclerosis. Neuroscience Bulletin, 29(2), 239–250.
Honma, Y., Araki, T., Gianino, S., Bruce, A., Heuckeroth, R., Johnson, E., et al. (2002). Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron, 35(2), 267–282.
Zappia, E., Casazza, S., Pedemonte, E., Benvenuto, F., Bonanni, I., Gerdoni, E., et al. (2005). Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood, 106, 1755–1761.
Acknowledgments
The authors are grateful to the Iranian Council of Stem Cell Technology, Isfahan University of Medical Sciences for their financial support (Grant No. 189067).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghasemi, N., Razavi, S., Mardani, M. et al. Transplantation of Human Adipose-Derived Stem Cells Enhances Remyelination in Lysolecithin-Induced Focal Demyelination of Rat Spinal Cord. Mol Biotechnol 56, 470–478 (2014). https://doi.org/10.1007/s12033-014-9744-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-014-9744-2