Abstract
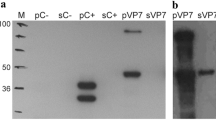
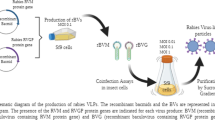
Bovine leukemia is a common retroviral infection of cattle. The disease is characterized by a strong immunological response to several viral proteins, but the antibodies against p24 and gp51 are predominant. In this study, a recombinant baculovirus containing the gag gene p24 was constructed and the protein, used as antigen, analyzed by western blot and an indirect in-house rp24-ELISA test. This allowed detecting the presence of antibodies for bovine leukemia virus in a panel of cattle sera. The authentication of the protein expands its potential use for different medical applications, from improved diagnosis of the disease to source of antigens to be included in a subunit vaccine.






Similar content being viewed by others
References
Burny, A., Bruck, C., Cleuter, Y., Couez, D., Deschamps, J., Gregoire, D., et al. (1985). Bovine leukemia virus and enzootic bovine leucosis. Onderstepoort Journal of Veterinary Research, 52(3), 133–144.
Burridge, M. J., Puhr, D. M., & Henneman, J. M. (1981). Prevalence of bovine leukemia virus infection in Florida. Journal of the American Veterinary Medical Association, 179, 704–707.
Hopkins, S. G., & DiGiacomo, R. F. (1997). Natural transmission of bovine leukemia virus in dairy and beef cattle. Veterinary Clinics of North America. Food Animal Practice, 13, 107–128.
Sagata, N., Yasunaga, T., Tsuzuku-Kawamura, J., Ohishi, K., Ogawa, Y., & Ikawa, Y. (1985). Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proceedings of the National Academy of Sciences of the United States of America, 82, 677–681.
Bex, F., Bruck, C., Mammerickx, M., Portetelle, D., Ghysdael, J., Cleuter, Y., et al. (1979). Humoral antibody response to bovine leukemia virus infection in cattle and sheep. Cancer Research, 39(3), 1118–1123.
Mammerickx, M., Portetelle, D., Burny, A., & Leunen, J. (1980). Detection by immunodiffusion- and radioimmunoassay-tests of antibodies to bovine leukemia virus antigens in sera of experimentally infected sheep and cattle. Zentralblatt für Veterinärmedizin. Reihe B, 27, 291–303.
De Boer, G. F., Boerrigter, H. M., Groen, J., & Osterhaus, A. D. (1987). Identification of bovine leukemia virus (BLV) infected cattle by complex-trapping-blocking (CTB) ELISA employing monoclonal antibodies directed against BLV-p24. Zentralblatt für Veterinärmedizin. Reihe B, 34, 717–728.
Kaaden, O. R., Lange, S., Romanowski, W., Marré, H., Pfeilsticker, J., & Roselius, R. (1982). Transient viraemia with bovine leukaemia virus in bulls. Zentralblatt für Veterinärmedizin. Reihe B, 29, 269–274.
Kittelberger, R., Reichel, M. P., Meynell, R. M., Tham, K. M., & Molloy, J. B. (1999). Detection of antibodies against the core protein p24 of the bovine leukaemia virus in cattle for confirmatory serological testing. Journal of Virological Methods, 77, 109–114.
Molloy, J. B., Walker, P. J., Baldock, F. C., Rodwell, B. J., & Cowley, J. A. (1990). An enzyme-linked immunosorbent assay for detection of bovine leukaemia virus p24 antibody in cattle. Journal of Virological Methods, 28, 47–57.
Rohrmann, G. F. (2008). Baculovirus molecular biology [Internet]. Bethesda, MD: National Center for Biotechnology Information (US). Available from: http://www.ncbi.nlm.nih.gov/books/NBK1736/.
Moscardi, F. (1999). Assessment of the application of baculoviruses for control of Lepidoptera. Annual Review of Entomology, 44, 257–289.
O’Reilly, D. R., Miller, L. K., & Luckow, V. A. (1992). Baculovirus expression vectors: A laboratory manual. New York: Freeman.
Hu, Y. C. (2005). Baculovirus as a highly efficient expression vector in insect and mammalian cells. Acta Pharmacologica Sinica, 26, 405–416.
Kost, T. A., Condreay, J. P., & Jarvis, D. L. (2005). Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nature Biotechnology, 23, 567–575.
Godeau, F., Saucier, C., & Kourilsky, P. (1992). Replication inhibition by nucleoside analogues of a recombinant Autographa californica multicapsid nuclear polyhedrosis virus harboring the herpes thymidine kinase gene driven by the IE-1(0) promoter: A new way to select recombinant baculoviruses. Nucleic Acids Research, 20, 6239–6246.
Lalumiere, M., & Richardson, C. D. (1995). Production of recombinant baculoviruses using rapid screening vectors that contain the gene for betagalactosidase. Methods in Molecular Biology, 39, 161–177.
Southern, J. A., Young, D. F., Heaney, F., Baumgärtner, W. K., & Randall, R. E. (1991). Identification of an epitope on the P and V proteins of simian virus 5 that distinguishes between two isolates with different biological characteristics. Journal of General Virology, 72, 1551–1557.
Gutiérrez, G., Alvarez, I., Fondevila, N., Politzki, R., Lomónaco, M., Rodríguez, S., et al. (2009). Detection of bovine leukemia virus specific antibodies using recombinant p24-ELISA. Veterinary Microbiology, 137, 224–234.
Trono, K. G., Pérez-Filgueira, D. M., Duffy, S., Borca, M. V., & Carrillo, C. (2001). Seroprevalence of bovine leukemia virus in dairy cattle in Argentina: Comparison of sensitivity and specificity of different detection methods. Veterinary Microbiology, 83, 235–248.
González, E. T., Oliva, G. A., Valera, A., Bonzo, E., Licursi, M., & Etcheverrigaray, M. E. (2001). Leucosis enzoótica bovina: Evaluación de técnicas de diagnóstico (ID, ELISA-I, WB, PCR) en bovinos inoculados experimentalmente. Analecta Veterinaria, 21, 12–20.
Van der Maaten, M., & Miller, J. M. (1990). Bovine leukosis virus. In Z. Dinter & B. Morein (Eds.), Virus infections of ruminants (pp. 419–429). Amsterdam, The Netherlands: Elsevier.
Bhatia, S., Patil, S. S., Sood, R., Dubey, R., Bhatia, A. K., Pattnaik, B., et al. (2008). Prokaryotic expression of a 750 bp capsid region of bovine immunodeficiency virus gag gene and development of a recombinant capsid (p26) protein based immunoassay for seroprevalence studies. Indian Journal of Biotechnology, 7, 34–45.
Costa, M., García, L., Yunus, A. S., Rockemann, D. D., Samal, S. K., & Cristina, J. (2000). Bovine respiratory syncytial virus: First serological evidence in Uruguay. Veterinary Research, 31, 241–246.
Bicka, L., Kuźmak, J., Kozaczyńska, B., Plucienniczak, A., & Skorupska, A. (2001). Expression of bovine leukemia virus protein p24 in Escherichia coli and its use in the immunoblotting assay. Acta Biochimica Polonica, 48, 227–232.
Juliarena, M., Gutierrez, S., & Ceriani, C. (2007). Chicken antibodies: A useful tool for antigen capture ELISA to detect bovine leukaemia virus without cross-reaction with other mammalian antibodies. Veterinary Research Communications, 31, 43–51.
Momtaz, H., Hemmatzadeh, F., & Keyvanfar, H. (2008). Expression of bovine leukemia virus p24 protein in bacterial cell. Pakistan Journal of Biological Sciences, 11, 2433–2437.
Zajac, V., & Sláviková, K. (1989). Expression of a bovine leukaemia virus envelope fusion protein in E. coli. Folia Biologica, 35, 35–41.
Dumont, J., Legrain, M., Portetelle, D., Brasseur, R., Burny, A., & Hilger, F. (1989). High yield synthesis of the bovine leukemia virus (BLV) p24 major internal protein in Saccharomyces cerevisiae. Gene, 79, 219–226.
Morton, C. L., & Potter, P. M. (2000). Comparison of Escherichia coli, Saccharomyces cerevisiae, Pichia pastoris, Spodoptera frugiperda, and COS7 cells for recombinant gene expression: Application to a rabbit liver carboxylesterase. Molecular Biotechnology, 16, 193–202.
Dube, S., Dolcini, G., Abbott, L., Mehta, S., Dube, D., Gutierrez, S., et al. (2000). The complete genomic sequence of a BLV strain from a Holstein cow from Argentina. Virology, 277, 379–386.
Amborski, G. F., Lo, J. L., & Seger, C. L. (1989). Serological detection of multiple retroviral infections in cattle: Bovine leukemia virus, bovine syncytial virus and bovine visna virus. Veterinary Microbiology, 20, 247–253.
Heeney, J., Valli, V., & Montesanti, J. (1988). Alteration in humoral immune response to bovine leukaemia virus antigens in cattle with lymphoma. Journal of General Virology, 69, 659–666.
Valker, P., Molloy, J., & Rodwell, B. (1987). A protein immunoblot test for detection of bovine leukemia virus p24 antibody in cattle and experimentally infected sheep. Journal of Virological Methods, 15, 201–211.
Bünger, I., Khalaf, H., Cripe, C., & Rimpler, M. (1994). Detection of antibodies against the virus of enzootic bovine leukosis in serum and milk samples using an immunoblot. DTW. Deutsche Tierarztliche Wochenschrift, 101, 402–405.
Grover, Y., & Guillemain, B. (1992). An immunoblotting procedure for detection of antibodies against bovine leukemia virus in cattle. Journal of Veterinary Medicine, 39, 48–52.
Merza, M., Sundquist, B., Söber, J., & Morein, B. (1991). Immunoaffinity purification of two major proteins of bovine leukemia virus (gp51 and p24) and their use for discrimination between vaccinated and infected animals. Journal of Virological Methods, 33, 345–353.
Acknowledgments
This work was supported by the Faculty of Veterinary Sciences of La Plata University, Argentina. The authors thank Dr Don Jarvis and Christoph Geisler from Wyoming University, USA for their help in the baculovirus expression system.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Larsen, A., Gonzalez, E.T., Serena, M.S. et al. Expression of p24 gag Protein of Bovine Leukemia Virus in Insect Cells and Its Use in Immunodetection of the Disease. Mol Biotechnol 54, 475–483 (2013). https://doi.org/10.1007/s12033-012-9587-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-012-9587-7