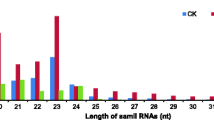
Abstract
To identify novel as well as conserved miRNAs in citrus, deep sequencing of small RNA library combined with microarray was performed in precocious trifoliate orange (an early flowering mutant of trifoliate orange, Poncirus trifoliata L. Raf.), resulting in the obtainment of a total of 114 conserved miRNAs belonging to 38 families and 155 novel miRNAs. The miRNA star sequences of 39 conserved miRNAs and 27 novel miRNAs were also discovered among newly identified miRNAs, providing additional evidence for the existence of miRNAs. Through degradome sequencing, 172 and 149 genes were identified as targets of conserved miRNAs and novel miRNAs, respectively. GO and KEGG annotation revealed that high ranked miRNA-target genes were those implicated in biological and metabolic processes. To characterize those miRNAs expressed at the juvenile and adult development stages of citrus, further analysis on the expression profiles of these miRNAs through hybridizing the commercial microarray and real-time PCR was performed. The results revealed that some miRNAs were down-regulated at adult stage compared with juvenile stage. Detailed comparison of the expression patterns of some miRNAs and corresponding target genes revealed the negative correlation between them, while few of them are positively correlated.






Similar content being viewed by others
References
Bartel, D. P. (2004). MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell, 116, 281–297.
Bartel, D. P., & Chen, C. Z. (2004). Micromanagers of gene expression: The potentially widespread influence of metazoan microRNAs. Nat Rev Genet, 5, 396–400.
Miranda, K. C., Huynh, T., Tay, Y., Ang, Y. S., Tam, W. L., et al. (2006). A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell, 126, 1203–1217.
Lanet, E., Delannoy, E., Sormani, R., Floris, M., Brodersen, P., et al. (2009). Biochemical evidence for translational repression by Arabidopsis microRNAs. Plant Cell, 21, 1762.
Brodersen, P., Sakvarelidze-Achard, L., Bruun-Rasmussen, M., Dunoyer, P., Yamamoto, Y. Y., et al. (2008). Widespread translational inhibition by plant miRNAs and siRNAs. Science, 320, 1185.
Baker, C. C., Sieber, P., Wellmer, F., & Meyerowitz, E. M. (2005). The early extra petals1 mutant uncovers a role for microRNA miR164c in regulating petal number in Arabidopsis. Current Biology, 15, 303–315.
Guo, H. S., Xie, Q., Fei, J. F., & Chua, N. H. (2005). MicroRNA directs mRNA cleavage of the transcription factor NAC1 to downregulate auxin signals for Arabidopsis lateral root development. Plant Cell, 17, 1376–1386.
Floyd, S. K., & Bowman, J. L. (2004). Gene regulation: Ancient microRNA target sequences in plants. Nature, 428, 485–486.
Chen, X. (2004). A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science, 303, 2022–2025.
Lee, R. C., Feinbaum, R. L., & Ambros, V. (1993). The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell, 75, 843–854.
Reinhart, B. J., Slack, F. J., Basson, M., Pasquinelli, A. E., Bettinger, J. C., et al. (2000). The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature, 403, 901–906.
Wu, G., & Poethig, R. S. (2006). Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development, 133, 3539.
Wu, G., Park, M. Y., Conway, S. R., Wang, J. W., Weigel, D., et al. (2009). The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell, 138, 750–759.
Wang, J. W., Schwab, R., Czech, B., Mica, E., & Weigel, D. (2008). Dual effects of miR156-targeted SPL genes and CYP78A5/KLUH on plastochron length and organ size in Arabidopsis thaliana. Plant Cell, 20, 1231–1243.
Wang, J. W., Czech, B., & Weigel, D. (2009). miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell, 138, 738–749.
Chuck, G., Meeley, R., Irish, E., Sakai, H., & Hake, S. (2007). The maize tasselseed4 microRNA controls sex determination and meristem cell fate by targeting Tasselseed6/indeterminate spikelet1. Nature Genetics, 39, 1517–1521.
Lauter, N., Kampani, A., Carlson, S., Goebel, M., & Moose, S. P. (2005). microRNA172 down-regulates glossy15 to promote vegetative phase change in maize. Proceedings of the National Academy of Sciences of the United States of America, 102, 9412–9417.
Zhang, J. Z., Li, Z. M., Yao, J. L., & Hu, C. G. (2009). Identification of flowering-related genes between early flowering trifoliate orange mutant and wild-type trifoliate orange (Poncirus trifoliata L. Raf.) by suppression subtraction hybridization (SSH) and macroarray. Gene., 430, 95–104.
Liang, S., Wang, X., & Wan, T. (1999). Precocious trifoliate orange (Poncirus trifoliata L. Raf.) biology characteristic and its stock experiment. ZheJiang Citrus, 19, 2–4. in Chinese.
Zhang, J. Z., Ai, X. Y., Sun, L. M., Zhang, D. L., Guo, W. W., et al. (2011). Molecular cloning and functional characterization of genes associated with flowering in citrus using an early-flowering trifoliate orange (Poncirus trifoliata L. Raf.) mutant. Plant Molecular Biology, 76(1–2), 187–204.
Kwak, P. B., Wang, Q. Q., Chen, X. S., Qiu, C. X., & Yang, Z. M. (2009). Enrichment of a set of microRNAs during the cotton fiber development. BMC Genomics, 10, 457.
Zhang, J., Xu, Y., Huan, Q., & Chong, K. (2009). Deep sequencing of Brachypodium small RNAs at the global genome level identifies microRNAs involved in cold stress response. BMC Genomics, 10, 449.
Rajagopalan, R., Vaucheret, H., Trejo, J., & Bartel, D. P. (2006). A diverse and evolutionarily fluid set of microRNAs in Arabidopsis thaliana. Genes & Development, 20, 3407–3425.
Lu, C., Tej, S. S., Luo, S., Haudenschild, C. D., Meyers, B. C., et al. (2005). Elucidation of the small RNA component of the transcriptome. Science, 309, 1567–1569.
Glazov, E. A., Cottee, P. A., Barris, W. C., Moore, R. J., Dalrymple, B. P., et al. (2008). A microRNA catalog of the developing chicken embryo identified by a deep sequencing approach. Genome Research, 18, 957.
Xu, Q., Liu, Y., Zhu, A., Wu, X., Ye, J., et al. (2010). Discovery and comparative profiling of microRNAs in a sweet orange red-flesh mutant and its wild type. BMC Genomics, 11, 246.
Song, C., Fang, J., Li, X., Liu, H., & Thomas Chao, C. (2009). Identification and characterization of 27 conserved microRNAs in citrus. Planta, 230, 671–685.
Zhang, J., Ai, X. Y., Sun, L. M., Zhang, D., Guo, W. W., et al. (2011). Transcriptome profile analysis of flowering molecular processes of early flowering trifoliate orange mutant and the wild-type [Poncirus trifoliata (L.) Raf.] by massively parallel signature sequencing. BMC Genomics, 12, 63.
Chen, X., Ba, Y., Ma, L., Cai, X., Yin, Y., et al. (2008). Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Research, 18, 997–1006.
Huang, J., Hao, P., Chen, H., Hu, W., Yan, Q., et al. (2009). Genome-wide identification of Schistosoma japonicum microRNAs using a deep-sequencing approach. PLoS One, 4, e8206.
Zuker, M. (2003). Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Research, 31, 3406.
Hofacker, I. L., Fontana, W., Stadler, P. F., Bonhoeffer, L. S., Tacker, M., et al. (1994). Fast folding and comparison of RNA secondary structures. Monatshefte für Chemie/Chemical Monthly, 125, 167–188.
Meyers, B. C., Axtell, M. J., Bartel, B., Bartel, D. P., Baulcombe, D., et al. (2008). Criteria for annotation of plant microRNAs. Plant Cell, 20, 3186–3190.
Thomson, J. M., Parker, J., Perou, C. M., & Hammond, S. M. (2004). A custom microarray platform for analysis of microRNA gene expression. Nature Methods, 1, 47–53.
Yang, Y. H., Dudoit, S., Luu, P., Lin, D. M., Peng, V., et al. (2002). Normalization for cDNA microarray data: A robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Research, 30, e15.
Gautier, L., Cope, L., Bolstad, B. M., & Irizarry, R. A. (2004). affy—Analysis of Affymetrix GeneChip data at the probe level. Bioinformatics, 20, 307.
Chen, C., Ridzon, D. A., Broomer, A. J., Zhou, Z., Lee, D. H., et al. (2005). Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Research, 33, e179.
Addo-Quaye, C., Eshoo, T. W., Bartel, D. P., & Axtell, M. J. (2008). Endogenous siRNA and miRNA targets identified by sequencing of the Arabidopsis degradome. Current Biology, 18, 758–762.
Addo-Quaye, C., Miller, W., & Axtell, M. J. (2009). CleaveLand: A pipeline for using degradome data to find cleaved small RNA targets. Bioinformatics, 25, 130.
Schmid, R., & Blaxter, M. L. (2008). annot 8 r: GO, EC and KEGG annotation of EST datasets. BMC Bioinformatics, 9, 180.
Ashburner, M., Ball, C. A., Blake, J. A., Botstein, D., Butler, H., et al. (2000). Gene ontology: Tool for the unification of biology. Nature Genetics, 25, 25–29.
Li, R., Yu, C., Li, Y., Lam, T. W., Yiu, S. M., et al. (2009). SOAP2: An improved ultrafast tool for short read alignment. Bioinformatics, 25, 1966.
Jones-Rhoades, M. W., Bartel, D. P., & Bartel, B. (2006). MicroRNAs and their regulatory roles in plants. Annual Review of Plant Physiology, 57, 19–53.
Song, C., Jia, Q., Fang, J., Li, F., Wang, C., et al. (2010). Computational identification of citrus microRNAs and target analysis in citrus expressed sequence tags. Plant Biology, 12, 927–934.
Wu, X. M., Liu, M. Y., Xu, Q., & Guo, W. W. (2010). Identification and characterization of microRNAs from citrus expressed sequence tags. Tree Genetics & Genomes, 1–17.
Song, C., Wang, C., Zhang, C., Korir, N. K., Yu, H., et al. (2010). Deep sequencing discovery of novel and conserved microRNAs in trifoliate orange (Citrus trifoliata). BMC Genomics, 11, 431.
Staiger, D., Allenbach, L., Salathia, N., Fiechter, V., Davis, S. J., et al. (2003). The Arabidopsis SRR1 gene mediates phyB signaling and is required for normal circadian clock function. Genes & Development, 17, 256–268.
Yao, Y., Guo, G., Ni, Z., Sunkar, R., Du, J., et al. (2007). Cloning and characterization of microRNAs from wheat (Triticum aestivum L.). Genome Biology, 8, R96.
Fahlgren, N., Howell, M. D., Kasschau, K. D., Chapman, E. J., Sullivan, C. M., et al. (2007). High-throughput sequencing of Arabidopsis microRNAs: Evidence for frequent birth and death of MIRNA genes. PLoS One, 2, e219.
Aukerman, M. J., & Sakai, H. (2003). Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell, 15, 2730–2741.
Moose, S. P., & Sisco, P. H. (1996). Glossy15, an APETALA2-like gene from maize that regulates leaf epidermal cell identity. Genes & Development, 10, 3018–3027.
Stone, J. M., Liang, X., Nekl, E. R., & Stiers, J. J. (2005). Arabidopsis AtSPL14, a plant-specific SBP-domain transcription factor, participates in plant development and sensitivity to fumonisin B1. Plant Journal, 41, 744–754.
Cardon, G. H., H hmann, S., Nettesheim, K., Saedler, H., & Huijser, P. (2002). Functional analysis of the Arabidopsis thaliana SBP-box gene SPL3: A novel gene involved in the floral transition. Plant Journal, 12, 367–377.
Jung, J. H., Seo, Y. H., Seo, P. J., Reyes, J. L., Yun, J., et al. (2007). The GIGANTEA-regulated microRNA172 mediates photoperiodic flowering independent of CONSTANS in Arabidopsis. Plant Cell, 19, 2736.
Acknowledgments
We are grateful to Prof. Han-Hui Kuang for his helpful discussion and help in revising this manuscript. This research was supported financially by the National Natural Science Foundation of China (grant nos. 30971973, 31071777, 30921002). The Science Foundation of the Doctoral Subject Point of the Chinese Ministry of Education (grant no. 20090146110009), the Fundamental Research Funds for the Central Universities (2011QC037, 2010BQ044), and the 863 Project of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s12033-011-9455-x.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, JZ., Ai, XY., Guo, WW. et al. Identification of miRNAs and Their Target Genes Using Deep Sequencing and Degradome Analysis in Trifoliate Orange [Poncirus trifoliate (L.) Raf]. Mol Biotechnol 51, 44–57 (2012). https://doi.org/10.1007/s12033-011-9439-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-011-9439-x