Abstract
We have previously described the development and implementation of a strategy for production of recombinant polyclonal antibodies (rpAb) in single batches employing CHO cells generated by site-specific integration, the SympressTM I technology. The SympressTM I technology is implemented at industrial scale, supporting a phase II clinical development program. Production of recombinant proteins by site-specific integration, which is based on incorporation of a single copy of the gene of interest, makes the SympressTM I technology best suited to support niche indications. To improve titers while maintaining a cost-efficient, highly reproducible single-batch manufacturing mode, we have evaluated a number of different approaches. The most successful results were obtained using random integration in a new producer cell termed ECHO, a CHO DG44 cell derivative engineered for improved productivity at Symphogen. This new expression process is termed the SympressTM II technology. Here we describe proof-of-principle data demonstrating the feasibility of the SympressTM II technology for single-batch rpAb manufacturing using two model systems each composed of six target-specific antibodies. The compositional stability and the batch-to-batch reproducibility of rpAb produced by the ECHO cells were at least as good as observed previously using site-specific integration technology. Furthermore, the new process had a significant titer increase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plasma-derived immunoglobulins have been used for passive immunotherapy for more than a century [1]. Current use of this type of products includes: treatment of infections with hepatitis B virus, cytomegalovirus, and rabies virus, while plasma-derived immunoglobulin containing anti-Rhesus D antibody is in use for prevention of hemolytic disease of the newborn and treatment of idiopathic thrombocytopenic purpura [2–4]. Plasma-derived immunoglobulins are, however, associated with several disadvantages such as low efficacy due to a low concentration of antigen-specific antibodies and potential safety issues due to the risk of disease transmission. Target-specific recombinant polyclonal antibodies (rpAb) would potentially overcome these problems [5–7].
Monoclonal antibodies, the second generation of antibody therapeutics, have been in medical use since the late 1980s. Monoclonal antibodies overcome the above-mentioned shortfalls of plasma-derived immunoglobulins, but they are less effective against diseases where a complex antigen is the cause [8]. Because monoclonal antibodies bind to only one single structure out of many on the surface of a complex antigen, they are less likely to be able to completely neutralize or eliminate that antigen.
Methods for development and manufacture of recombinant monoclonal antibodies are well established [9–11]. However, a new strategy is required to allow adaptation of these methods for consistent production of rpAb without substantially increasing the costs of production and regulatory approval.
Target-specific rpAb is a new generation of antibodies mimicking the diversity, specificity, and binding capability of the natural human immune system. To do this an rpAb preparation will often contain several specific antibodies, and for economic reasons a manufacturing procedure with all antibodies being produced in the same culture vessel will be preferable. Obviously, the ability to maintain the same antibody composition between batches, the so-called batch-to-batch consistency, is crucial for getting rpAb approved for human therapeutic use. Maintaining batch-to-batch consistency is complicated by the fact that the method entails culturing of different cell lines with potentially different specific productivities and growth rates.
We have previously published a controlled method for production of rpAb using site-specific integration technology [12] to minimize differences in growth rate and productivity from genomic position effects [13]. This technology (named SympressTM I) is being used for the production of an rpAb against the RhD antigen consisting of 25 individual antibodies. This manufacturing strategy has proven to result in highly consistent rpAb compositions from batch-to-batch. The product Sym001 (Rozrolimupab) is presently in clinical phase II. While SympressTM I is well suited for manufacturing of anti-Rhesus D rpAb, where the drug product demands on a yearly basis are relatively small, certain indications with a high product demand (e.g., many cancers) require a higher production level. For this purpose we have tested a number of alternative approaches. The most successful results regarding productivity and batch-to-batch consistency were obtained using a random integration approach. The feasibility of this approach was demonstrated in a new producer cell termed ECHO, a CHO DG44 cell derivative engineered for improved productivity at Symphogen. Using this system we were able to improve productivity significantly in a fed batch manufacturing process and at the same time keep the polyclonal composition consistent from batch-to-batch.
Materials and Methods
Generation of the ECHO Cell Line
The mammalian expression cell line used is a derivative of the DHFR-negative Chinese Hamster Ovary (CHO) cell line DG44 kindly provided by Dr. Lawrence Chasin, Columbia University (also available from Gibco cat # 12613-014). The DG44 cells were transfected with a cDNA encoding the 13S version of the adenovirus type 5 transactivator E1A (NCBI accession no. AY339865) in the vector pcDNA3.1 + (Invitrogen). Transfectants were selected with Geneticin (Invitrogen) and single-cell cloned by limiting dilution. A clone showing a transient antibody expression level increased by a factor of 3 compared to the untransfected DG44 cell line was termed the ECHO cell line.
Antibody Expression Vector
The employed antibody expression vector is shown in Fig. 1a. Antibody heavy and light chain expression is driven by two identical bi-directional human CMV promoters. The mouse dihydrofolate reductase (DHFR) is used as selection marker. DHFR expression is coupled to expression of the antibody heavy chain by an encephalomyocarditis virus (EMCV) internal ribosome entry site (IRES).
a Mammalian expression vector used for expression of the anti-Vaccinia and anti-RSV antibodies. CMV human CMV promoters, VH antibody heavy chain variable region, IgG1 constant genomic DNA encoding the human IgG1 constant region, IRES + DHFR ECMV internal ribosome entry sequence + mouse DHFR cDNA, SV40 poly A the SV40 polyadenylation sequence, bla and Amp ampicillin resistance gene, pUC ori pUC origin of replication, bGH poly A bovine growth hormone polyadenylation sequence, LC antibody light chain cDNA. b Comparison of specific productivities in selected pools of DG44 and ECHO cells. DG44 cells and ECHO cells were transfected with three different anti-Vaccinia virus antibody constructs (Ab numbers refer to the numbers used in Fig. 2). Specific productivities were measured in selected pools
Antibodies
For initial proof-of-principle experiments six different IgG1 kappa antibodies directed against different Vaccinia virus surface proteins were isolated using the SymplexTM technology [14]. The genes encoding the variable heavy and light chains of the individual antibodies were each cloned into the mammalian cell expression vector shown in Fig. 1a. The antibodies are further described in WO 2007/065433.
For the second model system, six different respiratory syncytial virus (RSV) specific IgG1 kappa antibodies isolated from infected individuals using the SymplexTM technology and selected for reactivity with the RSV F- and G- surface proteins were used.
Stable Transfection of ECHO Cells and Single Cell Cloning by FACS
ECHO cells were seeded in T80 flasks at a density of 0.15 × 106 cells/flask in MEM alpha medium with nucleosides (MEMalpha + medium; Invitrogen) with 10% fetal calf serum (FCS) (Invitrogen). After 24 h the cells were transfected using Fugene6 (Roche) according to the manufacturer’s instructions.
Pools of transfectants were selected with 2 nM (for generation of anti-Vaccinia antibody-expressing ECHO cells) or 3 nM (for generation of anti-RSV antibody-expressing ECHO cells) methotrexate (MTX) in nucleoside-free medium supplied with 10% dialyzed FCS (MEMalpha-medium). The MTX-selected pools were single-cell cloned by FACS based on high cell surface expression of IgG. Briefly, cells were detached, stained with phycoerythrin-coated goat anti-human IgG (IM1626, Beckman Coulter) and single-cell sorted by gating the upper (with regard to Mean Fluorescence Intensity) 1–2% into 96-well plates containing 100 μl of a 1:1 mix of MEMalpha-medium to conditioned MEMalpha + medium from non-transfected ECHO cells. No MTX was added to the culture medium from this point. The 96-well plates were visually screened for the presence of single-cell clones and after 10–13 days supernatants were screened by IgG ELISA. ELISA values and visual inspection of the degree of confluence in the wells were used to select clones for further culture.
Selected clones were transferred and propagated in T80 flasks and specific productivities were measured by IgG ELISA. The results were used to select clones for cryo-preservation and adaptation to serum-free suspension culture.
Adaptation to Serum-free Suspension Culture in ProCHO4
Adherent cells were adapted to serum-free suspension culture in ProCHO4 medium (ProCHO4 (Lonza), 4 mM l-glutamine (Invitrogen), 1% non-essential amino acids (Invitrogen), anti-clumping agent 1:250 (Invitrogen)) in aerated 50 ml cell culture tubes (TPP). The tubes were placed in a shaker incubator at 37°C and 5% CO2 at a shaking speed of 200 rpm. The medium was changed or the cultures diluted as needed. When growth was vigorous (doubling time below 30–35 h) the cells were considered adapted, after which they were expanded and frozen.
Adaptation to Chemically Defined Medium
The anti-RSV ECHO clones in ProCHO4 medium were transferred to shaker flasks in chemically defined medium at a shaking speed of 100 rpm. Medium exchange or dilution of cultures was performed when needed. The adaptation lasted around 3 weeks and was considered complete when doubling times were stable and below 35 h.
Co-Cultivation of Six Anti-Vaccinia Clones
The anti-Vaccinia co-cultivation experiment was performed in aerated 50 ml culture tubes in a total volume of 10 ml. The experiment was started with a cell concentration of 0.3 × 106 cells/ml. The cultures were diluted to 0.3 × 106 cells/ml twice a week with 3 and 4 days intervals. Once a week samples for ELISA and cation exchange chromatography (CIEX) analysis were taken starting on day 4 and ending on day 35.
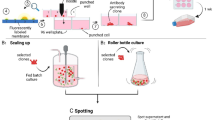
Preparation of Polyclonal Cell Banks
The two-tiered preparation of polyclonal cells banks is shown schematically in Fig. 4. Briefly, the chosen clones in logarithmic growth phase were mixed, aliquoted, and frozen in a number of ampoules (polyclonal master cell bank (pMCB)). Polyclonal working cell banks (pWCB) were prepared by thawing a pMCB ampoule, expanding the cells for a period, aliquoting and freezing a number of pWCB ampoules. The exact culture period at each step and the number of cells frozen differed from experiment to experiment (see “Results” section).
Bioreactor Fed Batch Productions
For the fed batch productions of the anti-Vaccinia rpAb compositions, one pWCB ampoule was thawed and transferred to ProCHO4 medium in a shaker flask and incubated at 37°C and 5% CO2. The cells were counted three times a week and diluted while expanding the volume as necessary. After 14 days, a 500 ml DASGIP bioreactor was inoculated with 0.6 × 106 cells per ml in 250 ml ProCHO4 medium at 80 rpm and 37°C. The culture was fed with ProCHO4 in a growth-rate-dependent manner up to 120 h, after which the feed was decreased gradually. Concentrations of glucose and l-glutamine were adjusted throughout, and sodium bicarbonate was used for pH adjustment. IgG product titer was determined by IgG specific HPLC. The cultivation was continued until the cell viability was below 70% (around 16 days) and the cells were removed from the supernatant by centrifugation and sterile filtration.
The fed batch productions of the anti-RSV rpAb compositions were performed in a similar manner, except that a chemically defined medium was used instead of the ProCHO4 medium and that a chemically defined feed was used instead of ProCHO4.
IgG ELISA
IgG was measured by sandwich ELISA. Briefly, 96-well plates (Maxisorp, NUNC) were coated with goat anti-human Fc (Serotec, STAR106) followed by incubation with samples and standard (a purified human monoclonal IgG1 kappa antibody). Detection was performed with goat anti-human kappa light chains conjugated with horseradish peroxidase (Serotec STAR100P).
Measurement of IgG Concentration by HPLC
Cell supernatants were centrifuged at 14,000×g, diluted 1:1 with PBS and sterile filtrated through a Millex-GV SLGV013SL Durapore 0.22 μm filter. Samples were run on a Poros A column (2.1 × 30 mm2, Applied Biosystems). 5–1000 μl were injected depending on the IgG concentration. Solvents: A Solvent: 1× PBS, B Solvent: 12 mM HCl; 150 mM NaCl, C Solvent: 20% Acetic Acid; 0.3 M MgCl2. System: Dionex HPLC Summit system with Pump p680—UVD170U detector—ASI-100 automated sample injector with 1000 μl loop. A purified monoclonal IgG1 kappa antibody was used as standard.
Purification
The anti-Vaccinia virus recombinant polyclonal antibodies were purified on a 1 ml MabSelect SuRe column (GE Healthcare) pre-equilibrated in PBS, pH 7.4. After loading with 5–10 ml 0.22 μm filtered medium supernatant, the column was washed with 10 ml PBS pH 7.4 and eluted with 0.1 M glycine, pH 2.7. The eluted product was dialyzed twice against 50 mM Na-acetate, 40 mM NaCl, pH 5.0. For the anti-RSV rpAb the same purification procedure was applied with a load of approximately 15 ml on a 50 μl self-packed column (Poly-Prep Column, Bio-Rad). The eluted product (250 μl) was adjusted to pH 7.0 using 4.5 μl 1.0 M Tris base.
Total IgG concentration was determined by measuring the absorbance at 280 nm.
CIEX Chromatography
The purified anti-Vaccinia virus rpAb samples were analyzed by CIEX on a PolyCat A column (4.6 × 100 mm2, 3 μm, 1500 Å from PolyLC Inc., Columbia, MD). The purified anti-RSV rpAb samples were analyzed by CIEX on a ProPac WCX-10 column (4 × 250 mm2 from Dionex). Both columns were equilibrated in 25 mM sodium acetate, pH 5.0, before application of 60 μg of the IgG mixture with a flow rate of 1 ml/min.
The anti-Vaccinia virus antibody peaks were subsequently eluted with a linear gradient from 150 to 500 mM NaCl in 25 mM sodium acetate, pH 5.0 over 44 CV. The anti-RSV antibody peaks were eluted with a linear gradient from 125 to 350 mM NaCl in 25 mM sodium acetate, pH 5.0, in 10 column volumes. The antibody peaks were detected spectrophotometrically at 215 nm. The chromatograms were peak integrated and the relative areas of the individual peaks determined.
Results
Derivation of the ECHO Cell Line
CHO DG44 cells were transfected with a cDNA for the adenoviral transactivator E1a and transfectants were selected with G418. Clones were screened in a transient transfection assay with an antibody expression construct. One clone showed consistently more than a factor 3 improvement in final antibody titer. This clone was subcloned twice and termed the ECHO cell line. Transient transfections with CMV-based EGFP constructs similarly showed increased expression in the ECHO cell line. Experiments aiming at identifying E1a transcripts by quantitative PCR in the cell line have been unsuccessful (not shown) so it is not clear whether E1a expression is mediating the higher expression or whether the cell has gained improved expression characteristics by random means during sub-cloning.
To investigate whether this improvement translated into an increased expression in stable cell lines we did a pool transfection experiment comparing the DG44 and ECHO cell lines. The cells were each transfected with three constructs encoding different antibodies against Vaccinia virus and transfectants were selected in nucleoside-free medium. Figure 1b shows specific productivities at the pool level indicating that the ECHO cell line gives productivities that are 3–5 times as high as for the DG44 cell line.
A number of antibody-expressing ECHO clones derived by the procedures described here were used for determination of copy number of the integrated construct by Southern blotting and quantitative PCR, and we typically found copy numbers above 100 (not shown).
Initial Co-Cultivation of ECHO Clone rpAb Compositions
With the aim to investigate whether different stable antibody-expressing ECHO cell clones generated by means of random integration could be cultivated together to produce an rpAb in a reproducible manner without skewing the composition or losing diversity, a model study was designed as follows: six different anti-Vaccinia antibodies were selected, each of them having a characteristic cation exchange chromatography (CIEX) profile distinguishable from the others. This facilitated identification and quantification of the six different antibodies in the compositions. ECHO cells were transfected individually with six different plasmids encoding anti-Vaccinia antibodies and selected for stable expression using the DHFR gene as selection marker. High-producing ECHO clones were isolated by surface labelling of the selected cell pool with fluorescent anti-IgG antibody followed by single-cell sorting by FACS of the 1–2% highest expressing cells into wells containing ECHO cell conditioned medium. Twenty four clones were selected for each antibody based on ELISA screening of production levels and visual inspection for growth. These 6 × 24 clones were tested in a 24-well medium exhaustion assay and the 10 highest producing clones for each antibody were adapted to serum-free ProCHO4 medium. Some clones were discarded during adaptation due to poor growth or decreasing productivity. When adaptation was finished 5–7 cell clones remained for each antibody. These were all frozen.
To test compositional stability of mixed cultures over a long time a number of ECHO clone compositions were prepared that would produce an rpAb consisting of the six different anti-Vaccinia antibodies. Based on cell counting during the late phase of the adaptation period clone doubling times were calculated and taken into consideration in the design of compositions to match clones with similar doubling time. Altogether nine compositions were prepared:
-
Compositions 1–5: Each antibody was represented by a single clone
-
Composition 6: Two clones were used for each antibody
-
Composition 7: Five clones were used for each antibody
-
Composition 8: Three clones were used for each antibody
-
Composition 9: All available clones were used, 5–7 for each antibody.
Clones were mixed so that the number of cells expressing each antibody (for each antibody 1–7 different ECHO clones) constituted 1/6 of the total number of cells in the mixed composition.
The idea behind the use of more than one clone for each antibody was that if a clone was lost due to poor growth there were replacement clones present which overall would increase the chance of having all antibodies represented in the final mix.
The cultivation experiment was performed for a period of 5 weeks. Once a week samples for ELISA and CIEX were taken. The cellular productivity as measured by ELISA was relatively constant over the 5 week period with a tendency toward a slight decline in some compositions (results not shown).
The relative content of the six individual antibodies was measured by CIEX at the start and end of cultivation of the nine rpAb cultures (Fig. 2). All antibodies could be detected in all nine cultures both at the start and the end of the experiment. In all cultures, changes in the amount of some of the individual antibodies were seen; in the final compositions individual antibodies constituted between 1.7 and 65%. There was not a clear tendency toward less skewing in composition in cultures with more than one ECHO clone representing each antibody (compositions 6–9 in Fig. 2).
Relative content of six anti-Vaccinia antibodies in nine compositions maintained in co-culture in 50 ml culture tubes for 6 weeks. The nine compositions containing six different anti-Vaccinia antibodies (I–VI) with one or more clonal ECHO cell line(s) expressing each antibody were prepared as described in “Materials and Methods” section. Supernatants were analyzed for content of the individual antibodies by CIEX at the start (dark gray columns) and end (light gray columns) of the cultures. The relative content of the six antibodies (along the horizontal axis) is given as percentage of total antibody (vertical axis)
The number of generations was between 25 and 27. This means that if the cultures had been expanded in full volume every time, the final total volumes would be 43,000–172,000 l. Large scale mammalian cell culture manufacturing processes in the industry are typically up to 15,000 l. It should however be emphasized that this initial experiment did not include cell banks, which means that fewer generations were needed from the first mixing of cells to the end point measurement compared to an industrial manufacturing process. Nevertheless, it was encouraging to find that the drift seen over this long period of co-culture was not more pronounced.
Based on these initial experiments a new set of experiments was designed: four new compositions were generated from clones producing the six antibodies replacing cell lines with unwanted behavior (pronounced drift up or down) with cell lines with desired growth characteristics. The compositions were 1A, 2A, 3A, and 4A of which 1A–3A contained one ECHO clone per antibody and 4A contained three ECHO clones per antibody. To better mimic a real manufacturing situation, polyclonal master and working cell banks (pMCBs and pWCBs) were prepared and a production phase was performed in bioreactors. The results showed that by removing clones with unwanted characteristics, the new compositions showed a less pronounced skewing of the antibody composition during the culture period. Furthermore, when the compositions were produced in duplicate using separate parallel production runs and starting seed-train expansions by thawing of different pWCB ampoules a high degree of batch-to-batch consistency resulted for all four compositions (Fig. 3).
Relative content of six anti-Vaccinia antibodies from two independent fed batch runs of each of the rpAb compositions 1A, 2A, 3A, and 4A, performed in DASGIP bioreactors at 500 ml scale as described in “Materials and Methods” section. The compositions 1A–3A contained one ECHO clone per antibody and 4A contained three ECHO clones per antibody. Samples for CIEX profile analysis were taken on day 16 of the bioreactor run
Overall, these first experiments were promising regarding the possibilities for producing rpAb in a reproducible manner using an iterative process for the selection of an appropriate composition of clones. The following experiments were initiated to confirm its usefulness using a different viral target.
Preparation of Anti-Respiratory Syncytial Virus rpAb Compositions
Next, the feasibility of the SympressTM II system was tested using a completely different rpAb composition, consisting of six antibodies all targeting the Respiratory Syncytial virus (RSV). For each of the six antibodies at least six different stable ECHO clones were generated and banked individually. Compositional stability studies with different combinations of the anti-RSV antibody-expressing ECHO clones were performed in suspension culture in chemically defined medium.
In order to mimic a large scale industrial manufacturing process studies were performed according to the scheme in Fig. 4. Different rpAb combinations of the anti-RSV ECHO clones were mixed and banked as pMCBs, followed by preparation of pWCBs. pWCB ampoules were thawed, seed-train expansion was performed in shaker flasks, and a production run was performed in 500 ml DASGIP bioreactors using a fed batch process as typically used also for mAb manufacturing. The studies were designed to simulate all the steps included in an industrial manufacturing process at 5,000–10,000 l scale as closely as possible. Thus, before banking of the pWCB as well as during seed-train expansion before inoculation of the bioreactor the cells were cultivated for the number of generations required to perform these steps in a scaled-up industrial process. The relative amounts of the individual antibodies in the rpAb compositions were analyzed by CIEX at the end of the experiment. An example of a CIEX profile of an anti-RSV composition is shown in Fig. 5. The single anti-RSV antibodies elute in distinct peaks and can be individually quantified by integration of the peak areas.
Experimental setup of the compositional stability studies with compositions expressing the anti-RSV rpAb. Suspension ECHO cell clones expressing each of the six monoclonal anti-RSV antibodies were banked individually. Selected ECHO clones, one for each of the six anti-RSV antibodies, were thawed and mixed. The mixed compositions were immediately frozen as polyclonal master cell banks (pMCB). One ampoule of the pMCB was subsequently thawed, expanded, and frozen as polyclonal working cell bank (pWCB). pWCB cells were then thawed and seed-train expanded prior to a fed batch production run in bioreactors
In an exploratory first set of compositional stability studies different compositions were prepared by combining clones with as similar doubling times as possible. Apart from testing compositions with one ECHO clone per antibody, we also tested a composition in which each antibody was represented by three different clones. The compositions were frozen as pMCBs, thawed again and expanded for an appropriate number of generations (>8) before preparation of pWCBs. Two pWCB ampoules were thawed for each composition to start parallel seed-trains. This was done in order to examine reproducibility of the production runs. Seed-train expansion was performed in shaker flasks. The relative antibody composition in the duplicate production runs was monitored by CIEX and the reproducibility of the duplicate runs was found to be acceptable.
However, in all compositions a tendency toward some antibodies dominating the cultures and other antibodies decreasing in quantity was observed (data not shown). The composition consisting of three ECHO clones per antibody resulted in a highly skewed composition after fed batch production. Based on these results, and because rpAb compositions prepared with one clone representing each antibody provides a simpler approach with respect to cell line characterization, it was decided to concentrate on one-clone-per-antibody compositions. The observations from the exploratory compositional study were used for preparation of new modified clone compositions (see below).
Optimized compositions were generated by replacing clones which had contributed to compositional instability by either taking over or disappearing from the culture with other clones showing better characteristics. Seed-trains and production runs were performed in duplicate for each composition after thawing of two different pWCB ampoules and following a seed-train and fed batch production scheme mimicking an industrial process at 5,000–10,000 l, as described above and in Fig. 4. In this set of experiments, several compositions were generated with an acceptable distribution of the individual antibodies after the production run. Also, the reproducibility of the parallel seed-trains and production runs was high (Fig. 6a, b).
Relative concentrations of the six different anti-RSV antibodies after single-batch manufacturing in fed batch mode. Three different combinations of stable ECHO clones expressing the anti-RSV rpAb were prepared and tested for compositional stability in a fed batch setup at 500 ml scale designed to simulate production at 5,000–10,000 l scale. The supernatant harvested after single-batch manufacturing was purified by protein A capture and the antibody compositions were analyzed by CIEX. The CIEX peaks were quantified by integration to obtain the relative distribution of the individual antibodies in the compositions. a Composition I, b composition II, c composition III. All runs were performed in duplicate
In a third round of compositional stability studies the information gained on the different ECHO clones in the two previous studies was used by combining clones with matching growth properties and productivities. The experiment was designed essentially as before. In this study compositions were generated with a more equal distribution of the antibodies after the production run (Fig. 6c). Here, the relative concentration of the single antibodies was between 12 and 25% at the end of the production run.
In order to confirm the batch-to-batch consistency we repeated seed-train expansion and fed batch production of composition I (Fig. 6a). The polyclonal seed-trains were run in four parallel shaker flasks and DASGIP bioreactors. The cells used for the four runs originated from four separately thawed ampoules of the pWCBs. Fed batch runs were as described before and CIEX profiles of supernatants were analyzed at day 10 of the fed batch process. As Fig. 7 indicates, the batch-to-batch variation within the four parallel runs was low. Also, the relative individual antibody concentrations in the supernatant harvested from these consistency runs were similar to the antibody concentrations obtained in the previous study. Slight differences might be explained by the different timing of sample taking (10 days vs. 12 days).
Comparison of the relative area of the individual anti-RSV antibodies from two independent sets of fed batch production runs performed with rpAb composition I. The dark bars represent the average antibody concentration of four parallel consistency runs. The light bars represent the average antibody concentration of the duplicate runs initially performed with composition I (see Fig. 6a). Samples for CIEX profiles were taken at day 10 (right bars) and day 12 (left bars). The standard deviation is indicated as error bars
Discussion
Here we describe the production of target-specific rpAb compositions exemplified by two completely different antibody compositions, namely an anti-Vaccinia virus rpAb and an anti-RSV rpAb, each comprised of six different IgG1 kappa antibodies specific for their intended target, using stable transfection of cells by random integration and production in a single-batch format. Surprisingly, the concept of using random integration turned out to be successful in both cases, which makes us suggest that the concept may be generally applicable for rpAb manufacturing. In bioreactor experiments simulating a 5,000–10,000 l production using extended seed-trains, the relative antibody compositions were very similar in parallel production runs. This indicates that batch-to-batch reproducibility, which is mandatory for regulatory approval of rpAbs as human therapeutics, is achieved by this manufacturing strategy. Furthermore, extrapolating the laboratory scale result to industrial manufacturing scale, the SympressTM II platform provides sufficient yields to support commercially sound cost-of-goods. Along with the SympressTM expression platform development a release and characterization strategy for regulatory approval of rpAb for clinical use have been established and several methods addressing the compositional variability of rpAb products have been established [3, Persson, P., Development of mass spectrometry based methods for identification and determination of compositional variability in recombinant polyclonal antibody products, unpublished results].
Having established a consistent rpAb batch-to-batch reproducibility, we wanted to explore to which extent the relative content of the antibodies in a composition could be controlled. For most indications one would envision that a 1:1:1:… antibody distribution would be beneficial, while other antibody ratios could be optimal for certain indications. Thus, with the goal to achieve a relatively equal distribution of the six antibodies in the final RSV rpAb composition an iterative approach where clones were mixed based on growth characteristics and productivity was shown to work. The data from the first compositions were used to exclude clones that behaved in an undesired manner, showing differential growth compared to the other clones or, alternatively, changing productivity over time. In new experiments it was possible to achieve compositions with a relatively equal distribution of the six antibodies after completion of the production phase.
It is well-known that CHO cell clones generated by random integration show a high variability in growth rate and productivity [15]. The production level and the specific protein to be expressed are also known to affect the clonal growth rate [16]. Polyclonal clone compositions will in an industrial setup have to go through at least 35 cell divisions in order to include two-tiered cell banking and seed-train expansion before reaching the final production phase. It was to be expected that cultivation for multiple generations of rpAb compositions expressing different antibodies at slightly different levels with potentially different growth rates would lead to one or more clones taking over the cultures and/or to clones disappearing. As we show here this is certainly seen to some extent, but surprisingly, a few iterations and exchange of clones with undesired characteristics made it possible to establish compositions where the rpAb composition is maintained in the final product. Importantly, the conceptual studies were designed to simulate a process appropriate for industrial manufacturing at large scale. The applied process includes a two-tiered cell banking step, which is important for a biological product for human therapeutic use in order to secure homogenous seed material for manufacturing throughout product lifespan.
Different alternatives could be thought of regarding production of rpAbs. First, all antibodies in a lead composition could be produced, purified, and characterized separately before being mixed in the final drug product. However, development costs would be high and most likely prohibitive if more than a few clones (3–4) should be present in the final composition. An alternative approach could be the use of partly separate culturing of the clones constituting the rpAb compositions: Monoclonal Master and Working cell banks (MCB and WCB) are prepared for the different clones constituting the final composition, and each clone is thawed and cultured individually during the whole seed-train or part of it. This would lead to a higher degree of control over the final composition since the number of generations in co-culture would be lower, although care would have to be taken to avoid microbial contamination or other manufacturing process irregularities in multiple cell lines growing in parallel under GMP conditions. Manufacturing scenarios using this approach are described in WO 2009/129814.
We have earlier reported the use of Flp-In site-specific integration for the generation of stable polyclonal producer cell banks exhibiting sufficiently similar growth and productivity properties to allow consistent single-batch manufacturing of rpAb [13]. Sym001 (Rozrolimupab), which comprises 25 anti-Rhesus D specific antibodies, is produced using this technology. At the time it was envisaged that the background for this consistency was the use of site-specific integration, which would serve to avoid genomic position effects with respect to growth properties of the cells, and at least to some extent result in equal expression levels. This technology where only one copy of the antibody gene is stably integrated into the expressing clone does however limit the production level of the stable producer cell lines. This makes the SympressTM I technology best suited to support niche indications. The modifications implemented in SympressTM II employing random integration in an improved cell line, a much higher copy number, use of a stringent DHFR selection system and implementation of FACS based sorting for high producers have led to a significant improvement in overall productivity (approximately 10-fold) while conserving the high batch-to-batch consistency observed with SympressTM I.
This makes the SympressTM II platform suitable for manufacturing of drug substance for major indications.
References
Casadevall, A., & Scharff, M. D. (1995). Return to the past: The case for antibody-based therapies in infectious diseases. Clinical Infectious Disease, 21, 150–161.
Hadley, A. G., Poole, G. D., Poole, J., Anderson, N. A., & Robson, M. (1996). Haemolytic disease of the newborn due to anti-G. Vox Sanguinis, 71, 108–112.
Scaradavou, A., Woo, B., Woloski, B. M. R., Cunningham-Rundles, C., Ettinger, L. J., Aledort, L. M., et al. (1997). Intravenous anti-D treatment of immune thrombocytopenic purpura: Experience in 272 patients. Blood, 89, 2689–2700.
Casadevall, A., Dadachova, E., & Pirofski, L. A. (2004). Passive antibody therapy for infectious diseases. Nature Reviews. Microbiology, 2, 695–703.
Sharon, T., Liebman, M. A., & Williams, B. R. (2005). Recombinant polyclonal antibodies for cancer therapy. Journal of Cellular Biochemistry, 96, 305–313.
Rasmussen, S. K., Rasmussen, L. K., Weilguny, D., & Tolstrup, A. B. (2007). Manufacture of recombinant polyclonal antibodies. Biotechnology Letters, 29, 845–852.
Haurum, J. S., & Bregenholt, S. (2005). Recombinant polyclonal antibodies: Therapeutic antibody technologies come full circle. IDrugs, 8, 404–409.
Cane, P. A. (2001). Molecular epidemiology of respiratory syncytial virus. Reviews in Medical Virology, 11, 103–116.
Chadd, H. E., & Chamow, S. M. (2001). Therapeutic antibody expression technology. Current Opinion in Biotechnology, 12, 188–194.
Kipriyanov, S. M., & Le Gall, F. (2004). Generation and production of engineered antibodies. Molecular Biotechnology, 26, 39–60.
Wurm, F. M. (2004). Production of recombinant protein therapeutics in cultivated mammalian cells. Nature Biotechnology, 22, 1393–1398.
O’Gorman, S., Fox, D. T., & Wahl, G. M. (1991). Recombinase-mediated gene activation and site-specific integration in mammalian cells. Science, 251, 1351–1355.
Wiberg, F. C., Rasmussen, S. K., Frandsen, T. P., Rasmussen, L. K., Tengbjerg, K., Coljee, V. W., et al. (2006). Production of target-specific recombinant human polyclonal antibodies in mammalian cells. Biotechnology and Bioengineering, 94, 396–405.
Meijer, P. J., Andersen, P. S., Haahr, H. M., Steinaa, L., Jensen, A., Lantto, J., et al. (2006). Isolation of human antibody repertoires with preservation of the natural heavy and light chain pairing. Journal of Molecular Biology, 358, 764–772.
Jones, D., Kroos, N., Anema, R., Van Montfort, B., Vooys, A., Van Der, K. S., et al. (2003). High-level expression of recombinant IgG in the human cell line per.c6. Biotechnology Progress, 19, 163–168.
Mazur, X., Fussenegger, M., Renner, W. A., & Bailey, J. E. (1998). Higher productivity of growth-arrested Chinese hamster ovary cells expressing the cyclin-dependent kinase inhibitor p27. Biotechnology Progress, 14, 705–713.
Acknowledgments
We thank NIH Grant# U01 AI 70378-03 for supporting the anti-Vaccinia antibody work. We are very grateful for the excellent technical assistance provided by Charlotte Berthelsen, Dorte Waaben, Hanne Wagner, Linda Jensen, Linea Viven Nørskov, Marianne Valløe Nielsen, Maria Schmidt-Larsen, Mikkel Færk Jacobsen, Ruth Lahoz, Tine Rudbeck, Yvonne Berger Larsen, and Winnie Listov-Saabye.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Nielsen, L.S., Baer, A., Müller, C. et al. Single-Batch Production of Recombinant Human Polyclonal Antibodies. Mol Biotechnol 45, 257–266 (2010). https://doi.org/10.1007/s12033-010-9270-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-010-9270-9