Abstract
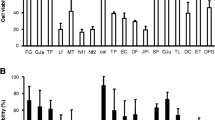
The aim of the present study was to evaluate the efficacy of novel nonviral gene delivery systems in cells of musculoskeletal origin. Primary cultures of lapine skeletal muscle cells, lapine articular chondrocytes, human cells from fibrous dysplasia and cell lines established from human osteosarcoma (SAOS-2), chondrosarcoma (CS-1), murine skeletal myoblasts (L8) and fibroblasts (NIH 3T3) were transfected with the P. pyralis luc or the E. coli lacZ genes using Nanofectin 1 and 2, Superfect, JetPEI, GeneJammer, Effectene, TransPass D2, FuGENE 6, Lipofectamine 2000, Dreamfect, Metafectene, Escort III, and calcium phosphate. Maximal transfection efficiency in lapine skeletal muscle cells was of 60.8 ± 21.2% using Dreamfect, 38.9 ± 5.0% in articular chondrocytes using Gene Jammer, 5.2 ± 8.0% in human cells from fibrous dysplasia using Lipofectamine 2000, 12.7 ± 16.2% in SAOS-2 cells using FuGENE 6, 29.9 ± 3.5% in CS-1 cells using Lipofectamine 2000, 70.7 ± 8.6% in L8 cells using FuGENE 6, and 48.9 ± 13.0% in NIH 3T3 cells using Metafectene. When the cells were transfected with a human IGF-I gene, significant amounts of the IGF-I protein were secreted. These results indicate that relatively high levels of transfection can be achieved using novel nonviral gene transfer methods.


Similar content being viewed by others
References
Evans, C. H., Ghivizzani, S. C., Herndon, J. H., & Robbins, P. D. (2005). Gene therapy for the treatment of musculoskeletal diseases. Journal of the American Academy of Orthopaedic Surgeons, 13, 230–242.
Luo, D., & Saltzman, W. M. (2000). Synthetic DNA delivery systems. Nature Biotechnology, 18, 33–37.
Madry, H., & Trippel, B. (2000). Efficient lipid-mediated gene transfer to articular chondrocytes. Gene Therapy, 7, 286–291.
Pampinella, F., Lechardeur, D., Zanetti, E., MacLachlan, I., Benharouga, M., Lukacs, G. L., & Vitiello, L. (2002). Analysis of differential lipofection efficiency in primary and established myoblasts. Molecular Therapy, 5, 161–169.
Hudde, T., Rayner, S. A., Comer, R. M., Weber, M., Isaacs, J. D., Waldmann, H., Larkin, D. F., & Gearge, A. J. (1999). Activated polyamidoamine dendrimers, a non-viral vector for gene transfer to the corneal endothelium. Gene Therapy, 6, 939–943.
Chemin, I., Moradpour, D., Wieland, S., Offensperger, W. B., Walter, E., Behr, J. P., & Blum, H. E. (1998). Liver-directed gene transfer: A linear polyethlenimine derivative mediates highly efficient DNA delivery to primary hepatocytes in vitro and in vivo. Journal of Viral Hepatitis, 5, 369–375.
Templeton, N. S., & Lasic, D. D. (1999). New directions in liposome gene delivery. Molecular Biotechnology, 11, 175–180.
Ravi Kumar, M., Hellermann, G., Lockey, R. F., & Mohapatra, S. S. (2004). Nanoparticle-mediated gene delivery: State of the art. Expert Opinion on Biological Therapy, 4, 1213–1224.
Madry, H., Kaul, G., Cucchiarini, M., Stein, U., Zurakowski, D., Remberger, K., Menger, M. D., Kohn, D., & Trippel, S. B. (2005). Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I). Gene Therapy, 12, 1171–1179.
Graham, F. L., & van der Erb, A. J. (1973). A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology, 52, 456–467.
Albert, N., & Tremblay, J. P. (1992). Evaluation of various gene transfection methods into human myoblast clones. Transplantation Proceedings, 24, 2784–2786.
Endesfelder, S., Bucher, S., Kliche, A., Rezska, R., & Speer, A. (2003). Transfection of normal primary human skeletal myoblasts with p21 and p57 antisense oligonucleotides to improve their proliferation: A first step towards an alternative molecular therapy approach of Duchenne muscular dystrophy. Journal of Molecular Medicine, 81, 355–362.
Dodds, E., Dunckley, M. G., Naujoks, K., Michaelis U., & Dickson, G. (1998). Lipofection of cultured mouse muscle cells: A direct comparison of Lipofectamine and DOSPER. Gene Therapy, 5, 542–551.
Trivedi, R. A., & Dickson, G. (1995). Liposome-mediated gene transfer into normal and dystrophin-deficient mouse myoblasts. Journal of Neurochemistry, 64, 2230–2238.
el Oakley, R. M., Brand, N. J., Burton, P. B., Cullen, M. C., Adams, G. B., Poznansky, M. C., Barton, P. J., & Yacoub, M. H. (1998). Efficiency of a high-titer retroviral vector for gene transfer into skeletal myoblasts. Journal of Thoracic and Cardiovascular Surgery, 115, 1–8.
Nabel, E. G., Yang, Z. Y., Plautz, G., Forough, R., Zhan, X., Haudenschild, C. C., Maciag, T., & Nabel, G. J. (1993). Recombinant fibroblast growth factor-1 promotes intimal hyperplasia and angiogenesis in arteries in vivo. Nature, 362, 844–846.
Marcellus, R. C., Teodoro, J. G., Charbonneau, R., Shore, G. C., & Branton, P. E. (1996). Expression of p53 in Saos-2 osteosarcoma cells induces apoptosis which can be inhibited by Bcl-2 or the adenovirus E1B-55 kDa protein. Cell Growth and Differentiation, 7, 1643–1650.
Marit, G., Cao, Y., Froussard, P., Ripoche, J., Dupouy, M., Elandaloussie, A., Lacombe, F., Mahon, F. X., Keller, H., Pla, M., Reiffers, J., & Theze, J. (2000). Increased liposome-mediated gene transfer into haematopoietic cells grown in adhesion to stromal or fibroblast cell line monolayers. European Journal of Haematology, 64, 22–31.
Weiskirchen, R., Kneifel, J., Weiskirchen, S., van de Leur, E., Kunz, D., & Gressner, A.M. (2000) Comparative evaluation of gene delivery devices in primary cultures of rat hepatic stellate cells and rat myofibroblasts. BMC Cell Biol,1, 4.
Akita, H., Ito, R., Khalil, I. A., Futaki, S., & Harashima, H. (2004). Quantitative three-dimensional analysis of the intracellular trafficking of plasmid DNA transfected by a nonviral gene delivery system using confocal laser scanning microscopy. Molecular Therapy, 9, 443–451.
Hellgren, I., Drvota, V., Pieper, R., Enoksson, S., Blomberg, P., Islam, K. B., & Sylvén, C. (2000). Highly efficient cell-mediated gene transfer using non-viral vectors and FuGene6: In vitro and in vivo studies. Cellular and Molecular Life Sciences, 57, 1326–1333.
Schakman, O., Gilson, H., de Coninck, V., Lause, P., Verniers, J., Havaux, X., Ketelslegers, J. M., & Thissen, J. P. (2005). Insulin-like growth factor-I gene transfer by electroporation prevents skeletal muscle atrophy in glucocorticoid-treated rats. Endocrinology, 146, 1789–1797.
Jeschke, M. G., Herndon, D. N., Baer, W., Barrow, R. E., & Jauch, K. W. (2001). Possibilities of non-viral gene transfer to improve cutaneous wound healing. Current Gene Therapy, 1, 267–278.
Gelse, K., von der Mark, K., Aigner, T., Park, J., & Schneider, H. (2003). Articular cartilage repair by gene therapy using growth factor-producing mesenchymal cells. Arthritis and Rheumatism, 48, 430–441.
Madry, H., Zurakowski, D., & Trippel, S. B. (2001). Overexpression of human insulin-like growth factor-I promotes new tissue formation in an ex vivo model of articular chondrocyte transplantation. Gene Therapy, 8, 1443–1449.
Romagnolo, D., Akers, R. M., Wong, E. A., Boyle, P. L., McFadden, T. B., & Turner, J. D. (1992). Overexpression of ovine insulin-like growth factor-I stimulates autonomous autocrine or paracrine growth in bovine mammary-derived epithelial cells. Molecular Endocrinology, 6, 1774–1780.
Eming, S. A., Snow, R. G., Yarmush, M. L., & Morgan, J. R. (1996). Targeted expression of insulin-like growth factor to human keratinocytes: Modification of the autocrine control of keratinocyte proliferation. Journal of Investigative Dermatology, 107, 113–120.
Acknowledgments
We thank L. Weissbach and F. Hornicek for providing the CS-1 cells, V. Flockerzi for the NIH 3T3 cells and Y. Mehraein for providing the SAOS-2 cells. H. Madry and M. Cucchiarini have been supported by grants from the Deutsche Forschungsgemeinschaft (DFG MA 2363/1-3 and DFG CU 55/1-3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orth, P., Weimer, A., Kaul, G. et al. Analysis of Novel Nonviral Gene Transfer Systems for Gene Delivery to Cells of the Musculoskeletal System. Mol Biotechnol 38, 137–144 (2008). https://doi.org/10.1007/s12033-007-0071-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-007-0071-8